Three dimensions sphere formaldehyde nanosensor applications: preparation and sensing properties
Abstract
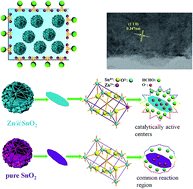
Zn@SnO2 microspheres with hierarchical structure were prepared through a simple solvothermal method; the structure and morphology were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), and high resolution transmission electron microscopy (HRTEM) showing the materials with extraordinary 3D nanoarchitectures. The gas sensing properties of the as-prepared pure SnO2 and Zn-doped SnO2 were tested toward various gases. The results showed that the SnO2 sensor with 6.67 wt% Zn-doping displayed an excellent selectivity toward formaldehyde at the operating temperature 160 °C, which was considerably lower than most formaldehyde sensors in heater type among previous reports, in addition to giving a response of about 15.2 to 100 ppm, which is about 2.1 times higher than that of sensors based on pure SnO2. The τres and the τrec values of the 6.67 wt% Zn-doped SnO2 sensor to 100 ppm formaldehyde were 2 s and 2 s respectively, demonstrating extraordinary gas sensing properties, whereas those of the pure SnO2 sensor were relatively long. The enhancement might be attributed to the unique morphology and increased oxygen vacancy due to the Zn doping.

 Please wait while we load your content...
Please wait while we load your content...