Influence of the carbon nanotube surface modification on the microstructure of thermoplastic binders
Abstract
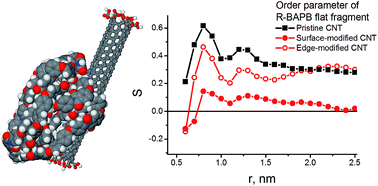
The structural properties of polymer nanocomposites based on thermoplastic polyimides filled with surface-modified carbon nanotubes (CNT) have been studied by means of fully-atomistic molecular-dynamics simulations. The influence of the distribution of functional carboxyl groups over the CNT surface on the polymer-matrix density distribution, and the orientational ordering of polymer chains have been investigated. It was shown that the polymer shifts far away from the nanoparticle surface with increase of the CNT modification degree. The orientational ordering of PI chains was not observed in the case of nanocomposites filled with modified CNTs where carboxyl groups are distributed uniformly on the surface. However, in case of the edge-modified CNTs the polymer can interact with the CNT surface; such edge-modified nanoparticles induce orientational ordering of crystallisable polyimide chains which can be considered as an initial stage of the polymer matrix crystallization.

 Please wait while we load your content...
Please wait while we load your content...