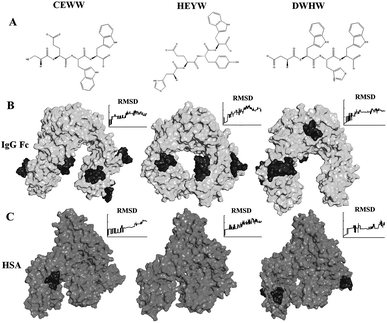
Development of novel small peptide ligands for antibody purification†
Yuping Weiab,
Jiandong Xua,
Liang Zhangab,
Yankai Fuab and
Xia Xu*a
aState, Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, P. R. China. E-mail: xuxia@ipe.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, P. R. China
First published on 23rd July 2015
Abstract
The huge demand for the most promising biopharmaceuticals, monoclonal antibodies (mAb), has resulted in a need for more efficient and less costly downstream purification processes for mAb. The currently used Protein A Agarose is unattractive due to toxic ligand leakage and high cost. In this study, three novel small peptide ligands, DWHW, CEWW and HEYW, were designed based on the interactions with human immunoglobulin G (IgG) using molecular simulations. The effects of pH, ionic strength and flow rate on the binding capacities to IgG were investigated. The static and dynamic binding capacities were determined. The dissociation constant (Kd) of the DWHW resin was 1.1 × 10−5 M. The binding capacity of the DWHW resin was 24.5 mg ml−1, which is comparable to Protein A Agarose. The DWHW resin was able to purify IgG from cMEM and CHO cell culture supernatants with a purity of more than 95% under elution conditions of pH 9 (50 mM sodium borate buffer) and pH 3 (50 mM glycine–HCl buffer). The results indicate that the small peptide ligands, especially DWHW, can offer a potential alternative for mAb purification.
1. Introduction
In recent years the huge demand for antibodies, the most promising biopharmaceuticals for the treatment of a number of diseases,1–3 has resulted in a need for a more efficient, less costly and more specific downstream production process to separate and purify antibodies from serum and mammalian cell cultures, which currently accounts for 50–80% of the overall production costs.4 Chromatography, including size-exclusion chromatography, hydrophobic interaction chromatography, ion-exchange chromatography and affinity chromatography has been trialled for the downstream purification process to purify antibodies on the industrial scale.5–7 Currently affinity chromatography using Protein A or Protein G as the affinity ligand is the most widespread method for monoclonal antibody (mAb) purification.4,8 However, highly purified Protein A and Protein G from bacterial sources are expensive, may lose their activities from harsh elution and sterilization conditions and can cause immunogenic responses due to their leakage.4,9 Therefore, there is a need to develop an alternative for antibody purification.A significant amount of work has been done in searching for an alternative for antibody purification instead of Protein A. Until now a number of alternatives have been identified, such as dyes,10,11 Protein A mimetic ligands,9,12 synthetic ligands13,14 and mixed-mode ligands.15,16 Since small peptide ligands have advantages over other ligands, such as high stability, relatively low toxicity, low cost and long lifetime even in harsh elution conditions, over the last two decades attempts have been made to design new small peptide ligands to replace Protein A for antibody purification. A number of small peptide ligands, including the hydroxybenzyl based ligands D2AAG and DAAG,16 the mimetic peptide (RTY)4K2KG,17,18 the ligands HYFKFD, HDRRHL and HWRGWV selected using a peptide library19,20 and the Protein A sequence-based peptide FYWHCLDE,21 have been generated using high throughput screening methods combined with molecular modeling. To our knowledge, no small peptide ligands for antibody purification have managed to stay on the market due to their relatively low binding capacity, selectivity and stability.21,36 Hence, it is necessary to develop novel small peptide ligands with high selectivity and stability, and low toxicity and cost for both small- and large-scale purification of antibodies.22,36
To develop small peptide ligands with high selectivity, no immunogenic responses and easy elution, a new strategy based on molecular simulations was established here. Due to the potential immune responses caused by the leakage of peptide ligands with more than 5 residues,37 ligands with less than 5 residues were designed using molecular simulations through the evaluation of the interactions between the ligands and the target receptor, the Fc fragment of the antibody. The free binding energy between the peptide ligands and the Fc fragment was evaluated using AutoDock Vina. We further investigated the molecular recognition between the Fc fragment and the lead peptide ligands in an aqueous environment using Gromacs. Here we present three small peptide ligands DWHW, CEWW and HEYW. The effects of NaCl concentration, pH and flow rate on their binding capacities to IgG were investigated. The dissociation constant (Kd) and maximum binding capacities (qm) of the ligands to IgG were determined using the Langmuir isotherm model. The dynamic binding capacities were also determined. The purity and recovery during the purification of human IgG from cMEM and CHO cell culture supernatants were measured and benchmarked against the commercial product Protein A Agarose using chromatographic evaluation.
2. Experimental
2.1 Materials
All fluorenylmethyloxycarbonyl (Fmoc) amino acids for solid-phase synthesis were purchased from GL Biochem. Ltd (Shanghai, China). The reagents for the peptide synthesis and the coupling agents were purchased from Aladdin (China), including O-(benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium tetrafluoro-borate (TBTU), N,N-dimethylformamide (DMF), 1-hydroxybenzotriazole (HOBt), ethyldi-isopropylamine (DIEA), isopropanol, 1,2-ethanedithiol (EDT), triisopropylsilane (TIS), piperidine and trifluoroacetic acid (TFA). All the sodium salts, acids and alkalis were obtained from Sinopharm Chemical Reagent (China). All the solvents were analytical grade. AF Amino-650M resin was obtained from Tosoh Bioscience (Japan). Protein A Agarose was obtained from Agarose Bead Technologies (Spain). Human IgG (IgG) and serum albumin (HSA) were purchased from Sino Biological Inc. (China). CHO cell culture supernatants containing mAb were obtained from a biopharmaceutical company that asked for confidentiality.2.2 Molecular simulations
The structure of human IgG (1FCC) and HSA (1BM0) were downloaded from the RCSB protein data bank (PDB). The structure of the polypeptides was sketched using the Discovery Studio Visualizer 3.0 (DSV). The molecular docking for the system of the peptide–Fc fragment was carried out using the AutoDock Vina software package, which significantly improves the average accuracy and speed of molecular docking.23–25 The AutoDock tool was used to prepare the ligands and receptors, the number of docking modes was set to 20 within a 80 Å × 90 Å × 60 Å grid box (grid spacing 1.0 Å) and other settings were set as the defaults. The potential candidates binding to IgG were selected based on the following criteria: (1) energy criteria – the top-scoring docking solutions with the lowest estimated binding free energy; (2) geometry criteria – the interaction site between a ligand and a receptor is not in the inner cavities of IgG.The molecular dynamics simulations were carried out using the Gromacs 4.5.4 simulation package.26 The simulations for the system of peptides–protein were performed for 5 ns using the standard Gromos96 G43a1 force field in combination with the Simple Point Charge (Extended) (SPCE) water model. System stability was verified by analyzing the root mean square deviation (RMSD) of the structures in the trajectory compared to the starting conformation. The simulation results were visualized using the DSV. All simulations were performed on an E30 workstation (Lenovo, China).
2.3 Peptide synthesis
All peptides were synthesized using the Fmoc coupling solid-phase synthesis strategy. Fmoc-6-aminocaproic acid (0.1 × 3 mmol g−1 resin) was firstly coupled to the resin as a space arm in a reaction mixture of TBTU (910 mg), HOBt (0.45 g), DIEA (0.52 ml) and DMF (10 ml) at room temperature for 2 h, followed by deprotection of the N-terminal Fmoc group with 25% piperidine in DMF. Other Fmoc amino acids were introduced in sequence. After each step, the resin was washed by isopropanol three times and then by DMF three times at room temperature. The degree of residue attachment after each step during the synthesis was evaluated by determining the absorbance of the removed Fmoc at 290 nm (Shanghai Spectrum, SP756P).38 After the addition of the last amino acid to the peptide chain, the protected group of the peptide side chain was cleaved in a solution containing TFA, H2O, EDT and TIS (94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5). Only the chromatography resins coupled with the peptides with a purity greater than 95% were used for further experiments.39
2.5). Only the chromatography resins coupled with the peptides with a purity greater than 95% were used for further experiments.39
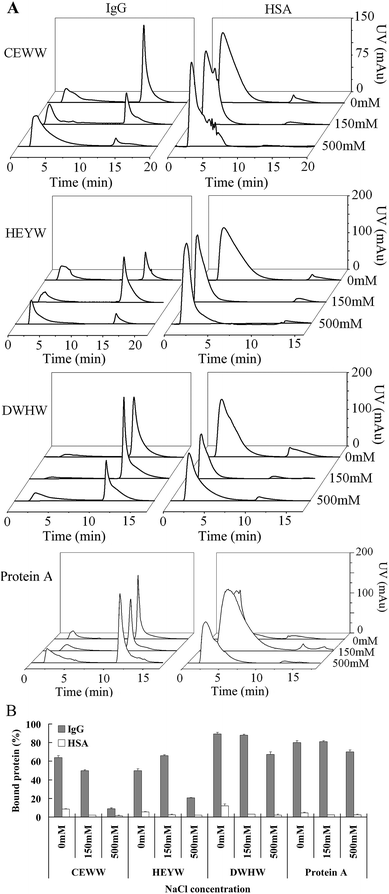
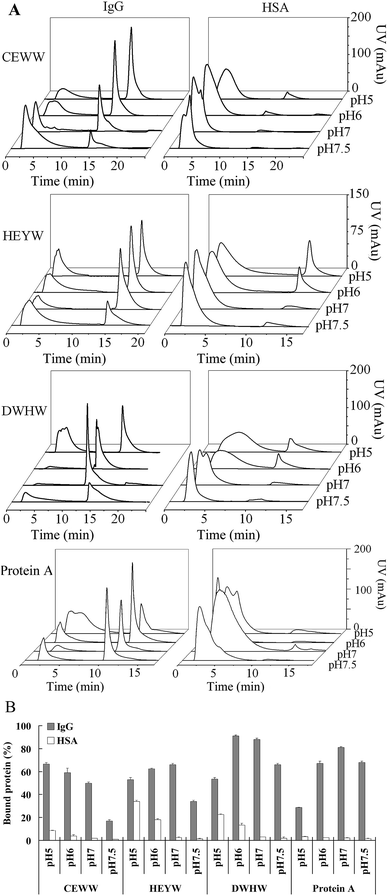
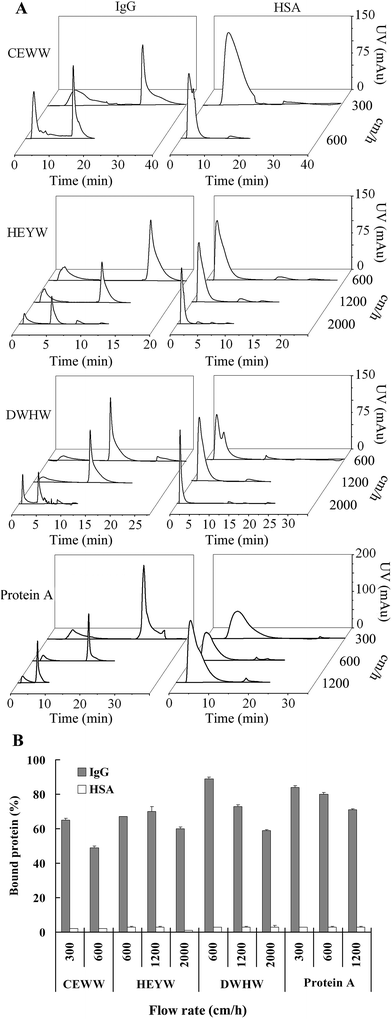
2.4 Chromatographic evaluation
The chromatographic evaluation was performed using an ÄKTA prime plus chromatographic system (GE Healthcare) with a 280 nm UV detector and a fraction collector. Resins coupled with the small peptide ligands and the commercial product of Protein A Agarose used as the chromatographic media were packed in a 150 μl (50 mm × 2 mm) column. The influence of NaCl concentrations in the equilibration and binding buffers on the binding capacities was measured at 0, 0.15 and 0.5 M NaCl at pH 7. The NaCl concentration in the equilibration and binding buffers was adjusted using 10 mM sodium phosphate (pH 7) buffer. Human IgG and HSA were diluted to 10 mg ml−1. After a pre-wash with the equilibration buffer, 100 μl IgG or HSA was loaded at a flow rate of 600 cm h−1. Then the column was washed with 10 column volumes (CVs) of binding buffer and then eluted with 5 CVs of elution buffer (50 mM sodium borate buffer, pH 9) at the same flow rate. The column was regenerated using 5 CVs of equilibration buffer. For the commercial product, Protein A Agarose media was eluted with 5 CVs of 50 mM glycine (Gly)–HCl buffer at pH 3. The effect of pH on the binding capacities was evaluated at pH 5, 6, 7 and 7.5. The equilibration and binding buffers containing 10 mM sodium phosphate and 150 mM sodium chloride were adjusted to the different pHs listed above. Then the same elution steps as above were followed. The effect of flow rate on the binding capacities was investigated at 300 cm h−1 (0.15 ml min−1), 600 cm h−1 (0.3 ml min−1), 1200 cm h−1 (0.6 ml min−1) and 2000 cm h−1 (1 ml min−1). After a pre-wash, 100 μl IgG or HSA was loaded at the flow rate of 600 cm h−1, 1200 cm h−1 and 2000 cm h−1. Then the elution steps as above were followed.2.5 Determination of the dynamic binding capacity
To determine the dynamic binding capacity of the peptides designed here, after a pre-wash with the equilibration buffer at pH 7, 10 mg IgG was loaded through a superloop at a flow rate of 600 cm h−1. Then the column was washed with 10 CVs of binding buffer and eluted with 5 CVs of elution buffer as previously described in section 2.4. 10% breakthrough testing to determine the dynamic binding capacity was carried out.2.6 Adsorption isotherm measurements
The adsorption isotherm measurements of the resins with the designed peptides and the commercial Protein A Agarose were performed in a 48-well plate at 25 °C. All the experiments were carried out at least twice. The 5 mg dry resins were equilibrated with the binding buffer at pH 7. 200 μl of IgG at different concentrations from 0.5 mg ml−1 to 8 mg ml−1 in PBS was added separately to the wells, and incubated on an orbital shaker at 400 rpm at room temperature for 2 h. The resins were removed by centrifugation and the concentration of the unbound IgG was measured with a UV detector at 280 nm (Shanghai, China). The amount of bound IgG was calculated according to the mass balance. The data was fitted to a Langmuir isotherm model:where q is the concentration of the bound proteins (mg protein per g resin), C is the concentration of the unbound proteins (mg protein per ml solution), Kd is the dissociation constant (mg ml−1) and qm is the maximum binding capacity (mg protein per g resin).
2.7 Chromatographic isolation of human IgG from cMEM and CHO cell culture supernatants
The resins coupled with the lead peptides and the commercial Protein A Agarose were used as the chromatographic media. The complete mammalian cell culture medium (cMEM) was formulated by combining mammalian cell culture medium (EMEM) with 10% fetal calf serum (FCS) and 5% tryptose phosphate broth (TPB). Human IgG was added into cMEM at a final concentration of 10 mg ml−1. To further determine the resin performance in a more conventional column, a 15 mm × 5 mm column was used for isolating human IgG from CHO cell culture supernatants. After a pre-wash with 10 CVs of equilibration buffer at pH 7, 100 μl of cMEM or 500 μl of CHO cell culture supernatants was injected at a flow rate of 600 cm h−1. Then the elution steps as previously described in section 2.4 were followed.2.8 Purity and recovery analysis
The purity of IgG in the collected fraction was analyzed using the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as previously described by Yang et al.19 under non-reducing conditions41 using 7.5% bis-tris gels on a single-sided vertical system equipped with an electrophoresis power supply (Tanon, China). Briefly, 20 μl of the sample with 5 μl loading buffer was boiled for 10 min and then added into the wells. The densitometry was performed through the determination of the gray scale of the bands in the Coomassie brilliant blue stained gels using the Image J software (National Institutes of Health, MD, USA). The purity of IgG was calculated as the ratio of the total area equivalent to the IgG at 150 kDa. The recovery was determined by calculating the ratio of the eluted IgG to the total loaded IgG.3. Results and discussion
3.1 Ligand design based on molecular simulations
Previous research has indicated that no more than four amino acids in Protein A provide major contributions to the interactions between IgG and Protein A, and the length of the interaction region between IgG and Protein A is less than 5 peptide lengths.27 Additionally, there are rare immune responses caused by the short peptides with less than 5 residues.37 Also a peptide with physiological toxicity usually contains 7–50 amino acids with a positive charge and a large proportion of Arg, Lys and hydrophobic residues.42,43 Thus, in this study, peptides with four residues or less not containing Arg and Lys were defined as the motif of small peptide ligands for antibody purification.The Kd, influenced by non-covalent intermolecular interactions, such as hydrogen bonding, electrostatic interaction, hydrophobic interactions and van der Waals forces, is commonly used to describe the affinity between a ligand and a receptor. The Kd is required to be in the range of 10−5 to 10−7 M for ligand design.28,29 Hence, based on the energy criteria and geometry criteria, only the peptides with a free binding energy less than −6.9 kcal mol−1 (Kd < 10−5 M) and interacting with the accessible sites of the Fc fragment surface were selected for further simulations. As is well known, to capture monoclonal antibodies from the culture medium containing 35–55 mg ml−1 of albumin, the ligands should not only have a high adsorption to the antibody, but also a low affinity to HSA. Hence, the adsorption of potential ligands to the Fc fragment and HSA was simulated with molecular dynamics simulations using Gromacs. The value of the RMSD is used to compare the structures in the trajectory with the starting conformation. As shown in Fig. 1, after 5 ns, little fluctuation of the RMSD during the molecular dynamics simulations indicates that the system reaches equilibrium. DWHW, CEWW and HEYW could interact with the Fc fragment selectively (ESI†). Table 1 shows the energy changes during the molecular dynamics simulations of DWHW, CEWW and HEYW binding to the Fc fragment. Both the coulomb and non-coulomb force contributed to the energy change, indicating that both hydrophobic interactions and electrostatic interactions affect the adsorption behaviors of these three peptides. The average energy changes of DWHW CEWW and HEYW were about 470 kJ mol−1, 303 kJ mol−1 and 298 kJ mol−1, respectively, indicating that DWHW may have a higher adsorption to the antibodies than the others. According to the above results, these three small peptide ligands showed excellent performance in both molecular docking and molecular dynamics simulations. Thus, DWHW, CEWW and HEYW were selected for further experiments to determine their binding capacities.
| Ligand | DWHW | CEWW | HEYW |
|---|---|---|---|
| Coulomb potential | −1091 | −689 | −455 |
| Non-coulomb potential | −789 | −827 | −440 |
| Total energy | −1880 | −1516 | −895 |
| Echange of single ligand | −470 | −298 | −303 |
3.2 Effect of ionic strength on the peptide adsorption of IgG and HSA
The selectivity and affinity of aromatic ligands towards proteins are mainly dependent on hydrophobic interactions.27 To investigate the effect of ionic strength on the binding capacities of three different peptide resins and Protein A Agarose, the NaCl concentration in the equilibration and binding buffers was adjusted to 0, 0.15 and 0.5 M. Fig. 2 shows the chromatograms and the percentage of IgG and HSA bound to the resins coupled with the three designed peptides. As seen in Fig. 2, the percentage of IgG bound to the CEWW resin dropped by around 80% when the NaCl concentration increased from 0 to 500 mM, while that for the DWHW resin dropped by around 30%, indicating that the CEWW peptide is more sensitive to the NaCl concentration than DWHW. The lower sensitivity of DWHW to the change in the NaCl concentration indicates that the binding of IgG to the DWHW resin is more specific and relies more on the hydrophobic interaction than that to the CEWW resin. These results also imply that the sequences of the small peptide ligands can influence the hydrophobic interaction and further affect the specific binding between the ligands and the protein. Additionally, the steric hindrance caused by the position of W in the peptides could be another reason for the lower sensitivity of DWHW compared to CEWW. As shown in Fig. 2, a reverse U shape during the IgG binding to the HEYW resin was observed. The increase in the concentration of NaCl from 0 to 150 mM led to the increase in the hydrophobic interaction and further resulted in the increase in the amount of IgG bound to the resin. However, a further increase in the NaCl concentration from 150 to 500 mM may cause changes in the conformation of IgG, especially the Fc fragment, and lead to shedding the accessible binding sites, eventually resulting in less IgG bound to the HEYW resin. In comparison with Protein A Agarose, the peptides designed here were more sensitive to the changes in the NaCl concentration, indicating that the selectivity and affinity of the peptides are more dependent on the hydrophobic interaction.Similarly to Protein A Agarose, the amount of HSA bound to the peptide resins decreased with the increase in the NaCl concentration (Fig. 2B), indicating that the binding between the peptide ligands and HSA is caused by the electrostatic interaction rather than by the hydrophobic interaction. Hence, the binding of HSA to the peptide ligands in the resins can be inhibited by the presence of NaCl. Additionally, the strange shapes of the chromatographic flow-through peaks in the HSA adsorption also imply that the HSA captured by the resins may be unstable and nonspecific.
3.3 Effect of pH on the peptide adsorption of IgG and HSA
Previous research has reported that the binding between Protein A and mAb is also dependent on the electrostatic interaction.27 Hence, here we determined the binding capacities of the peptide resins and Protein A Agarose to IgG and HSA at different pHs (pH 5, 6, 7 and 7.5), a critical factor for controlling the electrostatic interaction. Fig. 3 shows the chromatograms and the percentage of IgG and HSA bound to the resins with the three designed peptide ligands at different pHs in the equilibration and binding buffers. The result implies that the electrostatic interaction plays an important role in the IgG and HSA adsorption, especially for the peptide CEWW. The isoelectric point (pI) of CEWW, DWHW, HEYW, IgG and HSA is 3.2, 4.9, 5.1, 8 and 4.6, respectively. As seen in Fig. 3, the strong electrostatic interaction between CEWW and IgG at pH 5 led to an increase in the IgG adsorption from 16% at pH 7.5 to 66% at pH 5 while the weaker electrostatic interactions between the peptides DWHW and HEYW and IgG resulted in less IgG bound to the DWHW and HEYW resins at pH 5 than at pH 6 and pH 7. This might be the reason for the reverse U shape of the amount of IgG bound to the resins when the pH changes from pH 7.5 to pH 5. The pI of DWHW is very close to that of HEYW. However, different behaviors in the IgG adsorption were observed even at the same pH (Fig. 3). More IgG bound to the DWHW resin than the HEYW resin at the same pH implies that the electrostatic interaction is not the only factor affecting the interactions between these two small ligands and IgG, consistent with the simulation results (Table 1). This result further indicates that the hydrophobic interaction makes a significant contribution to the interaction between DWHW and IgG because there are more W in DWHW than in HEYW, which has been reported to selectively bind to the unconventional binding sites of IgG.30 Although the pI of Protein A is very close to that of HEYW and DWHW, much less IgG binding to Protein A Agarose (28.5%) than the peptide resins (51%) at pH 5 implies that the interaction between the small peptides and IgG is different from that between Protein A and IgG. At pH 5, the recognition site is probably blocked, further leading to a significant decrease in the IgG adsorption.44In contrast, it is not surprising to see that more HSA adsorbed to the DWHW and HEYW resins because the repulsive force between HSA and CEWW was greater than the force between HSA and DWHW and between HSA and HEYW at pH 5 and pH 6. With the increase of pH, no differences in the binding of HSA to the different resins were observed, probably because the repulsive force between HSA and the peptide ligands reached a similar level. Hence, the optimization of the pH in the equilibration and binding buffer can help to improve the selectivity of the CEWW, HEYW and DWHW resins, similarly to the Protein A Agarose.27
3.4 Effect of flow rate on the peptide adsorption of IgG and HSA
The flow rate is a critical factor for antibody purification operating in a high-performance mode, especially for large scale purification.31 Fig. 4 shows the chromatograms and the percentage of IgG and HSA bound to the resins coupled with the three designed peptide ligands and the Protein A Agarose at different flow rates of 300 cm h−1, 600 cm h−1, 1200 cm h−1 and 2000 cm h−1. Considering the selectivity and binding capacities of the peptide ligands to IgG and HSA at different NaCl concentrations and different pHs (Fig. 2 and 3), 150 mM of NaCl and a pH of 7 were used in the equilibration and binding buffers. As seen in Fig. 4B, the percentage of IgG bound to the DWHW resin at the flow rates of 600 cm h−1 and 1200 cm h−1 was much greater than that for the CEWW and HEYW resins at the corresponding flow rates, indicating that the specific binding of IgG to the DWHW resin was much stronger than the binding to the HEYW and CEWW resins, which was consistent with the molecular simulation results (Table 1). In comparison with the commercial Protein A Agarose, the binding capacity of the DWHW to IgG was similar at the flow rates of 600 and 1200 cm h−1. Even at the high flow rate of 2000 cm h−1, which the commercial Protein A Agarose cannot stand, around 59% of IgG was bound to the DWHW resin.The residence time is vital to enhance the speed of antibody purification, but a short residence time may cause a loss in the binding efficiency.32 Here, the behavior of IgG adsorbed to the DWHW resin was evaluated at flow rates of 600 cm h−1 to 2000 cm h−1 with residence times of 0.5 and 0.15 min, respectively. As seen in Fig. 4B, the percentage of the IgG bound to the DWHW resin was 10% greater than that for the commercial Protein A Agarose at 600 cm h−1, and was comparable to the commercial Protein A Agarose at 1200 cm h−1. Furthermore, the nonspecific binding of HSA was reduced with a short residence time at the fast flow rate (Fig. 4).
3.5 Determination of static binding capacities
The static adsorption at different IgG concentrations from 0.5 to 8 mg ml−1 was performed to obtain the Kd. The density of the ligand of the peptide resins was determined by absorbance of removed Fmoc group at 290 nm.38 The adsorption isotherms of the resins with the different peptides DWHW, CEWW and HEYW at a density of 95 μmol ml−1 and the commercial Protein A Agarose were measured at room temperature. Since the swelling ratio of the Toyopearl 650M material (4.7 ml g−1) is different from the commercial Protein A Agarose (4 ml g−1), the maximum binding capacities of the resins were calculated based on the amount of IgG adsorbed (mg) to the resins (g) in mg g−1 as described previously.19,20 All the static adsorption experiments were carried out at pH 7 (as indicated in Fig. 3). As shown in Fig. 5, the data was fitted to a Langmuir isotherm model. The Kd and the maximum capacity of each resin are listed in Table 2. | ||
| Fig. 5 Langmuir fits of the isotherms for IgG adsorption to CEWW, HEYW, DWHW resins and Protein A Agarose. | ||
| Ligand | Adsorption isotherms | ||
|---|---|---|---|
| Kd (×10−5 M) | qm (mg g−1) | R2 | |
| a Kd, the dissociation constant; qm, the maximum binding capacity. | |||
| DWHW | 1.1 | 95.2 | 0.995 |
| HEYW | 1.7 | 140.8 | 0.991 |
| CEWW | 1.2 | 117.6 | 0.959 |
| Protein A | 0.1 | 84.0 | 0.978 |
The maximum binding capacities of the DWHW, HEYW and CEWW resins at a ligand density of 95 μmol ml−1 were 95.2, 140.8 and 117.6 mg g−1, respectively, greater than that of the commercial Protein A Agarose with a maximum binding capacity of 84.0 mg g−1, and comparable to the maximum binding capacity of the hexamer peptide resin previously reported.16 The surface area of 1 g resins is 30 m2.14 Hence, the surface density of IgG bound to the peptide resins was in the range from 3.2 to 4.7 mg m−2, indicating that IgG molecules formed a monolayer on the surface of the ligand resins when reaching saturation in the binding buffer according to Yang et al.14 The Kd values for DWHW, CEWW and HEYW resins were of the same order of magnitude, in the range of 10−5 to 10−6 M, similar to the small peptide ligands previously reported.28,33 The Kd values of the peptide ligands were about one order of magnitude lower than those of the commercial Protein A Agarose (0.1 × 10−5 M) consistent with the previous reports,14,34 indicating that the small peptide ligands designed in this study might have less affinity to IgG than Protein A. However, it should be pointed out that the strong affinity provided by Protein A requires a harsh elution condition (pH 3) to release the captured IgG, which may lead to aggregation and denaturation of the IgG after elution. In contrast, a relatively mild elution condition could meet the requirements for releasing the captured IgG from the peptide ligand CEWW, DWHW and HEYW resins. Hence, the peptide ligands designed here with the Kd values of around 10−5 M are probably suitable for operation in the high-performance mode.35
3.6 Determination of dynamic binding capacities
The dynamic binding capacities of the three designed peptide resins and the commercial Protein A Agarose were investigated. The binding capacity of the commercial Protein A Agarose was 24.4 mg ml−1, consistent with the previous study.16 The binding capacities of HEYW and CEWW were 16.5 and 18.8 mg ml−1, at the same level as the hydroxybenzyl based D2AAG and DAAG peptide resins and the HWRGWV peptide resin.16 The binding capacity of DWHW was 24.5 mg ml−1, superior to that of the HWRGWV peptide resin and the commercial resin.14 It has been demonstrated that the binding capacity is related to the ligand density.14 At a high ligand density, due to steric hindrance, the functional groups for specific binding in the small peptide ligands may not be able to be exposed to IgG. This eventually results in a low capacity/density index for the DWHW, HEYW and CEWW resins compared with the previous study.16 Hence, the ligand density on the resin should be further optimized to improve the antibody purification performance.3.7 Purification of IgG from cMEM and CHO cell culture supernatants
The chromatographic evaluation and SDS-PAGE for the IgG isolated from cMEM and CHO cell culture supernatants using the peptide ligand resins and the commercial Protein A Agarose are shown in Fig. 6. The purities of IgG isolated by the peptide ligand resins and the commercial Protein A Agarose are listed in Table 3. The SDS-PAGE results showed that the IgG was successfully isolated from the cMEM by the peptide resins with a purity of more than 95% (Fig. 6A), indicating that the peptide resins can selectively capture IgG.The CEWW and HEYW resins showed a lower recovery than the DWHW resin and the commercial Protein A Agarose (Table 3). Although the purity of IgG isolated by the DWHW resin was slightly lower than that for the Protein A Agarose, more IgG was captured by the DWHW resin, resulting in a similar recovery (87% and 88.3% with the elution condition at pH 9 and pH 3, respectively) at a flow rate of 600 cm h−1 to the Protein A Agarose at a flow rate of 300 m h−1 (Table 2). Compared to the commercial products Protein A Sepharose CL-4B, Sepharose 4 Fast Flow (GE) and Protein A Agarose (ABT) with dynamic capacities of 20–30 mg ml−1,40 the processing time for isolating mAb using the DWHW resin can be shortened by half when a similar recovery rate is achieved. In comparison with other peptide ligands in previous reports,14,16 the DWHW resin showed a slight increase in both purity and yield at standard chromatographic conditions.19,20
For the purification of IgG from CHO cell culture supernatants (Fig. 6B), 1.5 mg ml−1 of IgG was injected. The DWHW, CEWW and HEYW resins were able to selectively capture mAb with purities of 95.2%, 95.8% and 98.7%, respectively (Table 3). Further calculation showed that a recovery of 84.6% was achieved using the DWHW resin, similar to that for the Protein A Agarose (85.4%), consistent with the previous studies.20 The purification of IgG from CHO cell culture supernatants was also carried out in a low aspect ratio column (15 mm × 5 mm). The SDS-PAGE results showed that the IgG was successfully isolated from the cMEM by the DWHW resin with a purity of around 95% (ESI†) and a recovery of around 83%. These results indicate that the performance of the DWHW resins is not affected by the aspect ratio of column. Besides, the IgG captured by the DWHW resin could be eluted at pH 9 and pH 3, whereas pH 3 was required by the Protein A Agarose. The elution condition could be further optimized to an even milder condition. Additionally, in this study the elution condition with a low ionic strength buffer allows us to avoid a desalination dialysis treatment after elution. Therefore, the small peptide ligand of DWHW could be a good potential ligand instead of Protein A for antibody purification.
4. Conclusions
To meet the huge demand for efficiently non-toxic and less costly downstream purification processes for mAb, three small peptides of DWHW, CEWW and HEYW were designed based on the energy criteria and geometry criteria using molecular simulations. The novel peptide ligands presented here, particularly the DWHW ligand, show promising results for IgG purification. Compared with the commercial Protein A Agarose, DWHW has a similar binding capacity (24.5 mg ml−1). The DWHW ligand is able to purify IgG from cMEM and CHO cell culture supernatants with a purity of more than 95% and a recovery of around 85% similar to the commercial Protein A Agarose. This novel small peptide of DWHW can offer a potential alternative for mAb purification with elution conditions at either pH 9 or pH 3 instead of Protein A at pH 3 to release the captured IgG easily.Acknowledgements
This research was supported by the Chinese Academy of Sciences, and the National Natural Science Foundation of China (21176238).References
- K. Huse, H.-J. Böhme and G. H. Scholz, J. Biochem. Biophys. Methods, 2002, 51, 217–231 CrossRef CAS
.
- P. Chames, M. van Regenmortel, E. Weiss and D. Baty, Br. J. Pharmacol., 2009, 157, 220–233 CrossRef CAS PubMed
.
- J. H. Chon and G. Zarbis-Papastoitsis, New Biotechnol., 2011, 28, 458–463 CrossRef CAS PubMed
.
- D. Low, R. O’Leary and N. S. Pujar, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2007, 848, 48–63 CrossRef CAS PubMed
.
- R. Necina, K. Amatschek and A. Jungbauer, Biotechnol. Bioeng., 1998, 60, 689–698 CrossRef CAS
.
- S. C. Goheen and R. S. Matson, J. Chromatogr. A, 1985, 326, 235–241 CrossRef CAS
.
- G. M. Tan, X. Y. Dong and Y. Sun, J. Sep. Sci., 2006, 29, 684–690 CrossRef CAS PubMed
.
- R. Hahn, R. Schlegel and A. Jungbauer, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2003, 790, 35–51 CrossRef CAS
.
- A. C. Roque, G. Gupta and C. R. Lowe, Methods Mol. Biol., 2005, 310, 43–62 CAS
.
- R. Wongchuphan, B. T. Tey, W. S. Tan, F. S. Taip, S. M. M. Kamal and T. C. Ling, Biochem. Eng. J., 2009, 45, 232–238 CrossRef CAS PubMed
.
- S. F. Teng, K. Sproule, A. Hussain and C. R. Lowe, J. Mol. Recognit., 1999, 12, 67–75 CrossRef CAS
.
- B. D’Agostino, P. Bellofiore, T. de Martino, C. Punzo, V. Rivieccio and A. Verdoliva, J. Immunol. Methods, 2008, 333, 126–138 CrossRef PubMed
.
- Z. Liu, P. V. Gurgel and R. G. Carbonell, J. Chromatogr. A, 2012, 1262, 169–179 CrossRef CAS PubMed
.
- H. Yang, P. V. Gurgel and R. G. Carbonell, J. Chromatogr. A, 2009, 1216, 910–918 CrossRef CAS PubMed
.
- Q. H. Shi, Z. Cheng and Y. Sun, J. Chromatogr. A, 2009, 1216, 6081–6087 CrossRef CAS PubMed
.
- L. N. Lund, P. E. Gustavsson, R. Michael, J. Lindgren, L. Norskov-Lauritsen, M. Lund, G. Houen, A. Staby and P. M. St Hilaire, J. Chromatogr. A, 2012, 1225, 158–167 CrossRef CAS PubMed
.
- A. Verdoliva, F. Pannone, M. Rossi, S. Catello and V. Manfredi, J. Immunol. Methods, 2002, 271, 77–88 CrossRef CAS
.
- G. Fassina, M. Ruvo, G. Palombo, A. Verdoliva and M. Marino, J. Biochem. Biophys. Methods, 2001, 49, 481–490 CrossRef CAS
.
- H. Yang, P. V. Gurgel and R. G. Carbonell, J. Pept. Res., 2005, 66(suppl. 1), 120–137 Search PubMed
.
- S. Menegatti, A. D. Naik, P. V. Gurgel and R. G. Carbonell, J. Chromatogr. A, 2012, 1245, 55–64 CrossRef CAS PubMed
.
- W.-W. Zhao, F.-F. Liu, Q.-H. Shi, X.-Y. Dong and Y. Sun, Biochem. Eng. J., 2014, 88, 1–11 CrossRef CAS PubMed
.
- C. R. Lowe, Curr. Opin. Chem. Biol., 2001, 5, 248–256 CrossRef CAS
.
- G. M. Morris, D. S. Goodsell, R. S. Halliday, R. Huey, W. E. Hart, R. K. Belew and A. J. Olson, J. Comput. Chem., 1998, 19, 1639–1662 CrossRef CAS
.
- R. Huey, G. M. Morris, A. J. Olson and D. S. Goodsell, J. Comput. Chem., 2007, 28, 1145–1152 CrossRef CAS PubMed
.
- O. Trott and A. J. Olson, J. Comput. Chem., 2010, 31, 455–461 CAS
.
- D. van der Spoel, E. Lindahl, B. Hess, G. Groenhof, A. E. Mark and H. J. Berendsen, J. Comput. Chem., 2005, 26, 1701–1718 CrossRef CAS PubMed
.
- B. Huang, F. F. Liu, X. Y. Dong and Y. Sun, J. Phys. Chem. B, 2011, 115, 4168–4176 CrossRef CAS PubMed
.
- L. Wang, G. Amphlett, W. A. Blattler, J. M. Lambert and W. Zhang, Protein Sci., 2005, 14, 2436–2446 CrossRef CAS PubMed
.
- N. J. Alves, S. D. Stimple, M. W. Handlogten, J. D. Ashley, T. Kiziltepe and B. Bilgicer, Anal. Chem., 2012, 84, 7721–7728 CrossRef CAS PubMed
.
- K. Rajagopalan, G. Pavlinkova, S. Levy, P. R. Pokkuluri, M. Schiffer, B. E. Haley and H. Kohler, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 6019–6024 CrossRef CAS
.
- B. Kelley, Biotechnol. Prog., 2007, 23, 995–1008 CAS
.
- R. Fahrner, H. Iyer and G. Blank, Bioprocess Eng., 1999, 21, 287–292 CrossRef CAS
.
- D. B. Kaufman, M. E. Hentsch, G. A. Baumbach, J. A. Buettner, C. A. Dadd, P. Y. Huang, D. J. Hammond and R. G. Carbonell, Biotechnol. Bioeng., 2002, 77, 278–289 CrossRef CAS PubMed
.
- S. Ghose, B. Hubbard and S. M. Cramer, J. Chromatogr. A, 2006, 1122, 144–152 CrossRef CAS PubMed
.
- R. Hatti-Kaul and B. Mattiasson, Isolation and purification of proteins, CRC Press, 2003 Search PubMed
.
- A. C. A. Roque, C. S. O. Silva and M. Â. Taipa, J. Chromatogr. A, 2007, 1160, 44–55 CrossRef CAS PubMed
.
- D. Kanduc, Adv. Exp. Med. Biol., 2008, 640, 198–207 CrossRef CAS
.
- W. Chan and P. D. White, Fmoc solid phase peptide synthesis: a practical approach, Oxford University Press, USA, 2000, pp. 62–63 Search PubMed
.
- T.-C. Chen, H. Waldmann and P. J. Fairchild, J. Clin. Invest., 2004, 113, 1754–1762 CrossRef CAS PubMed
.
- GE Life Sciences Antibody purification handbooks online, http://www.gelifesciences.com/webapp/wcs/stores/servlet/catalog/zh/GELifeSciences/service-and-support/handbooks/.
- A. J. Gearing, S. J. Thorpe, K. Miller, M. Mangan, P. G. Varley, T. Dudgeon, G. Ward, C. Turner and R. Thorpe, Immunol. Lett., 2002, 81, 41–48 CrossRef CAS
.
- D. Gaspar, A. S. Veiga and M. A. Castanho, Front. Microbiol., 2013, 4, 1–16 Search PubMed
.
- P. Bulet, C. Hetru, J.-L. Dimarcq and D. Hoffmann, Dev. Comp. Immunol., 1999, 23, 329–344 CrossRef CAS
.
- H. Watanabe, H. Matsumaru, A. Ooishi, Y. Feng, T. Odahara, K. Suto and S. Honda, J. Biol. Chem., 2009, 284, 12373–12383 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: The results of a molecular dynamics simulation about the interaction between the peptide ligands and the Fc region of IgG. See DOI: 10.1039/c5ra07829f |
| This journal is © The Royal Society of Chemistry 2015 |