Polytriazole bridged with 2,5-diphenyl-1,3,4-oxadiazole moieties: a highly sensitive and selective fluorescence chemosensor for Ag+†
Shoupeng Cao,
Zhichao Pei,
Yongqian Xu,
Ruina Zhang and
Yuxin Pei*
College of Science, Northwest A&F University, Yangling, Shaanxi 712100, People's Republic of China. E-mail: peiyx@nwafu.edu.cn; Fax: +86 2987092769; Tel: +86 2987091196
First published on 14th May 2015
Abstract
Fluorescent conjugated polytriazoles (FCP 1–4) containing both 2,5-diphenyl-1,3,5-oxadiazole (OXD) and 1,2,3-triazole moieties in the main chain were synthesized from aromatic diazide (1) and dialkynes (2–5) via click polymerization, respectively. In the polymers, OXDs (fluorophores) and triazole rings (generated via CuAAC acting as metal ion ligands) comprise a fluorescent system. The polytriazoles displayed relatively strong emission with quantum yields in the range of 0.20–0.28 at room temperature in DMF. The study on their ion-responsive properties showed that, although all four FCPs have good selectivity for Ag+, the integration of alkoxy side groups (methoxy for FCP 2, hexyloxy for FCP 3 and 2-ethylhexyloxy for FCP 4) to the main chains of the polytriazoles decreased their sensitivity for Ag+ via alteration of the polymer aggregation status and electron density of the main chains. Thus FCP 1 is highly sensitive for Ag+, where its Ksv is as high as 1.44 × 105 M−1 and its lowest detection limit is in the ppb range (4.22 × 10−7 M). This study provides an efficient click approach to the synthesis of a novel fluorescence sensor for Ag+ detection, which could expand the application of click polymerization in designing fluorescence sensors based on the triazole unit.
Introduction
The widespread applications of silver and its compounds in electric, photographic, and pharmaceutical industries have led to a large amount of silver being released into the environment from industrial wastes and emissions. As a highly toxic heavy metal ion, excess silver ions cause bioaccumulation and toxicity,1 inactivation of sulfhydryl enzymes,2 and irreversible darkening of the skin. In the past decades, the upsurge of silver nanoparticles (Ag-NPs), extensively applied in medicine, environmental remediation, and information technology, causes direct Ag-NPs release into environmental systems.3 In the presence of moisture, oxidation of Ag-NPs results in the release of Ag+ ions. The relative environmental concerns are therefore more pressing than ever, thus the development of selective and sensitive fluorescent chemosensors for Ag+ is highly desirable. To date, the majority of fluorescence sensors for Ag+ ions have been based on small or single molecule dyes.4–6 Few literatures have reported fluorescent sensors based on conjugate polymers for silver-ion detection.7–9Compared to small molecule sensors,10,11 fluorescent conjugated polymer chemosensors for metal ions are increasingly attractive to researchers due to their advantages from enhancements associated with electronic communication between receptors along polymer backbones, processability, and feasibility to tune the electronic structure to change or enhance the selectivity of the system.12
Click polymerization, which originates from click reaction coined by Sharpless in 2001,13 has become a powerful polymerization technique for new materials with tailored functionalities, where most often is carried out between di-or multiazides and di-or multialkynes with Cu(I) as catalyst (copper catalyzed alkyne-azide cycloaddition, CuAAC).14,15 Thus, polytriazoles (PAs, named after the formation of the unique structural units of N-containing five-member 1,2,3-triazole ring from azide-alkyne cycloaddition) have been synthesized as highly efficient fluorescent polymer liquid crystal,15 semiconductors,16 hyper-cross-linked fluorescent polymer nanoparticles for cell imaging,17 and highly sensitive fluorescent chemosensor for nitroaromatic explosives,18,19 by incorporating chosen functional groups. Most attractively, numerous 1,2,3-triazole structural units along polymer backbones can act as potentially versatile ligands for metal coordination20 and produce the “molecular wire effect”21 for sensing signal amplification when used as optical sensors for metal ions. Moreover, by using a clickable monomer containing a fluorophore, a fluorescent PA with high sensitivity can be obtained. This extraordinary property, arising from the large numbers of both binding sites and fluorophores, allows efficient interaction with the target ions or molecules. Hence, a few PA-based fluorescent sensors selective for Hg2+ were synthesized by Zhu and his coworkers.22–24 Incorporating benzo[2,1,3]thiadiazole moieties to PA, the same researchers successfully synthesized a fluorescent polymer for Ni2+ with detection limit as low as 1.1 × 10−7 M.25 However, to the best of our knowledge, there is no polytriazole fluorescent chemosensor for Ag+ reported.
On the other hand, 1,3,4-oxadiazole unit with electron-deficient nature is well known for their high electron affinity values and excellent electron-transporting properties, and is a very popular chromophore in polymers for photonic devices, such as OLEDs,26 polymer light-emitting diodes,27 and photovoltaic cells.28 Polymers containing 1,3,4-oxadiazole units endow the final polymer not only high thermo-stability and chemical stability, but also high photoluminescence efficiency.29 For instance, Cheng and his coworkers synthesized linear chiral polymers based on optically active polybinaphthyls and 1,3,4-oxadiazole, which showed strong green–blue fluorescence.30 Although the polymers showed excellent sensitivities when used as fluorescent chemosensor for metal ions, no selectivity was observed: the polymers showed similar quenching efficiencies for Ni2+, Cu2+, Pb2+, Zn2+, Hg2+, and Ag+. Moderate coordination ability of Ag+ and the strong interferes of Hg2+ and other heavy metal ions make Ag+ difficult to be discriminated from other chemically similar heavy metal ions.6,7 The design and synthesis of highly sensitive and selective system for the determination of trace amount of silver ions remain a challenge.
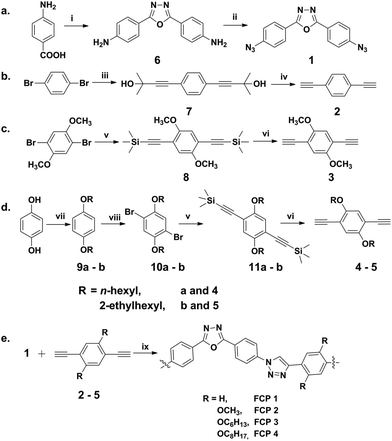
In this work, by using 2,5-bis(4-azidophenyl)-1,3,4-oxadiazole (1) as diazide monomer, and 1,4-diethynylbenzene (2), 1,4-diethynyl-2,5-bis(methoxyl)benzene (3), 1,4-diethynyl-2,5-bis(hexyloxy)benzene (4), and 1,4-diethynyl-2,5-bis(2-ethylhexyloxy)benzene (5) as dialkynyl monomers (Scheme 1), novel fluorescent polytriazoles containing both units of 1,2,3-triazole and 1,3,4-oxadiazole were first synthesized via click polymerization. Thereafter, the polytriazoles were characterized by FTIR, GPC, TGA, and DSC, respectively. Their fluorescence property, selectivity, and sensitivity for heavy metal ions were investigated. To our delight, we found that FCP 1 (fluorescent conjugated polytriazole formed with monomer 1 and 2) showed excellent selectivity and sensitivity for Ag+.
Results and discussion
To synthesize polytriazoles bridged with 2,5-diphenyl-1,3,4-oxadiazole (OXD) moieties via click polymerization, diazide monomer 2,5-bis(4-azidophenyl)-1,3,4-oxadiazole (1) and dialkyne monomers 1,4-diethynylbenzene (2), 1,4-diethynyl-2,5-bis(methoxyl)benzene (3), 1,4-diethynyl-2,5-bis(hexyloxy)benzene (4), and 1,4-diethynyl-2,5-bis(2-ethylhexyloxy)benzene (5) were obtained first according to the published procedures (Scheme 2a–d).31–37,55 Monomer 1 was designed to contain a 1,3,4-oxadiazole unit for incorporating it to the polymer backbone later via click polymerization. Clickable functional groups of azide and alkyne were chosen specifically for performing CuAAC reaction to generate 1,2,3-triazole units. Methoxyl, n-hexyloxy and 2-ethylhexyloxy groups were introduced to monomer 3, 4, and 5, respectively, to obtain polytriazoles with side chains in order to study their influence on solubility, aggregation, and fluorescent properties. Thus, via click reaction between azide and alkyne groups, four FCPs were synthesized (Scheme 2e): FCP 1 was from 1 and 2, FCP 2 from 1 and 3, FCP 3 from 1 and 4, FCP 4 from 1 and 5, respectively, in DMF in the presence of Cu(PPh3)3Br.15 Both units of oxadiazole and triazole were incorporated into the polymer backbones. After precipitation, washing, and drying steps, FCP 1, FCP 2, FCP 3, and FCP 4 were obtained with a yield of 37%, 49%, 61%, and 67%, respectively, as yellowish solids.It's worthy to mention that the residual copper in the final polymers from the catalyst Cu(PPh3)3Br used in the polymerization is rather low (roughly equal to 0.1 equiv.),38 the quenching caused by it is little and can be ignored (see Fig. S14†).
The polymers were characterized by GPC, FTIR, TGA, and DSC, respectively.
The number-average molecular weights (Mn), the weight-average molecular weights (Mw), and the polydispersity indexes (PDI) of FCPs were determined by GPC using polystyrene standards in DMF (Table 1). It can be seen that the polymers synthesized in this work have moderate molecular weights and acceptable PDIs: 3780 and 1.51 for FCP 1, 5240 and 1.32 for FCP 2, 11600 and 1.24 for FCP 3, and 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 1.50 for FCP 4. A noteworthy observation is the molecular weights of FCP 3 and 4, which are much higher than FCP 1 under the same polymerization conditions, potentially as a result of the synergetic effect of higher monomer reactivity of 4 and 5 due to the electron-donating bulky alkoxy groups at ortho-position of benzene ring and better solubility of comb oligomers formed during the polymerization processes.
000 and 1.50 for FCP 4. A noteworthy observation is the molecular weights of FCP 3 and 4, which are much higher than FCP 1 under the same polymerization conditions, potentially as a result of the synergetic effect of higher monomer reactivity of 4 and 5 due to the electron-donating bulky alkoxy groups at ortho-position of benzene ring and better solubility of comb oligomers formed during the polymerization processes.
| FCPs | Mn (×103) | Mw (×103) | PDI | Td (°C) |
|---|---|---|---|---|
| FCP 1 | 3.78 | 5.7 | 1.51 | 289 |
| FCP 2 | 5.24 | 6.9 | 1.32 | 204 |
| FCP 3 | 11.6 | 14.5 | 1.24 | 273 |
| FCP 4 | 12.0 | 18.0 | 1.50 | 239 |
The click polymerization between azide and alkynyl groups (monomer 1 and 2) were followed by NMR for 2 h. The 1H NMR spectra of the starting materials and the reaction mixture after 2 h were recorded and shown in Fig. S1.† It can be seen that a new peak appeared at 9.56 ppm, attributed to the proton in the new-generated triazole ring. In the meantime, the integration of the peak at 4.38 ppm (HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–) decreased with time, indicating a consumption of alkynyl groups with reaction and a successful click reaction between monomer 1 and 2.
C–) decreased with time, indicating a consumption of alkynyl groups with reaction and a successful click reaction between monomer 1 and 2.
The FTIR spectrum of FCP 1 is shown in Fig. 1. Compared to the IR spectra of monomer 1 and 2, a new absorption peak at 3106 cm−1 was observed, which is assigned to the stretching vibration of C–H on triazole ring formed in click polymerization.39 The sharp absorption band of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N
N–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in monomer 140 at 1490 cm−1 deformed slightly due to the stretching vibrations of C
C in monomer 140 at 1490 cm−1 deformed slightly due to the stretching vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, N
C, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, and triazole ring at 1508 cm−1.40–43 Although the stretching vibrations of C–H (3296 cm−1) in alkyne and azide (2092 cm−1) in the polymer were still observed, the relative intensities of azide to C
N, and triazole ring at 1508 cm−1.40–43 Although the stretching vibrations of C–H (3296 cm−1) in alkyne and azide (2092 cm−1) in the polymer were still observed, the relative intensities of azide to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N in OXD unit (1610 cm−1)40,41 and C
N in OXD unit (1610 cm−1)40,41 and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH decreased significantly. A similar phenomenon was also observed in the IR spectra of FCP 2, 3, and 4, though the intensity decrease was even more significant (Fig. S2–S4†) due to the lower percentages of the residual end groups of azide and alkyne and higher DPs (degrees of polymerization) in FCP 2, 3, and 4 compared to FCP 1 (see Table 1). All these results indicated a successful click reaction had taken place between monomer 1 and 2–5, respectively.
CH decreased significantly. A similar phenomenon was also observed in the IR spectra of FCP 2, 3, and 4, though the intensity decrease was even more significant (Fig. S2–S4†) due to the lower percentages of the residual end groups of azide and alkyne and higher DPs (degrees of polymerization) in FCP 2, 3, and 4 compared to FCP 1 (see Table 1). All these results indicated a successful click reaction had taken place between monomer 1 and 2–5, respectively.
Thermal properties of FCPs were evaluated by TGA and DSC under N2 atmosphere at a heating rate of 10 °C min−1. According to the thermograms from TGA (Fig. 2), the four polymers had similar thermal stabilities: 5% weight loss was observed at higher than 200 °C, and an apparently degradation was observed at temperature ranging from 240 °C to 650 °C. The temperatures for 5% weight loss (Td) of FCP 1–4 was 289, 204, 273 and 239 °C, respectively (Table 1). While two endothermic peaks centered at ∼180 and ∼320 °C were recorded on the DSC thermograms of the FCPs (Fig. S5†). We speculated that the peak at 180 °C could be attributed to the deterioration of small segments or terminal groups, corresponding to the temperature for less than 5% weight loss on TGA thermograms. Since the obvious weight loss of the FCPs occurred after 300 °C (see Fig. 2), it is thus reasonable to assign the second peak at ∼320 °C to the thermal decomposition of the polymer skeletons. The results showed that the polymers possess desirable thermal properties, which most likely can be attributed to the multi-ring structure on the main chains of the polymers, although the side groups (methoxyl for FCP 2, hexyloxy for FCP 3, and 2-ethylhexyloxy for FCP 4) impaired the thermostability of the polymers to some extent, as shown in Fig. 2 and S5.†
FCPs are nonfluorescent in solid state. However, under UV light (λex = 365 nm), FCPs showed blue–green fluorescence in common aprotic organic solvents such as dichloromethane, THF, chloroform, acetonitrile, DMF, and DMSO. While in protic solvent methanol, no fluorescence was observed. It is well-known that alcohols can form intermolecular hydrogen-bonded complex with small fluorescent molecules to produce profound effect on their fluorescent behavior.44 However, few reports were found to discuss the effect of methanol on emission of conjugated fluorescent polytriazoles (CPAs): Bunz and his co-workers reported that methanol had no alteration of the emission of poly(arylenetriazolyene)s, while addition of acid to the polymers caused a red shift, which was attributed by the authors to conformation changes by protonation of triazole rings.16 In the other study, a mixture of methanol/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) was used as solvent to dissolve CPA to investigate the quenching efficiency of Hg2+.24 Based on these reports, it is interesting to further investigate the fluorescent behaviour of FCPs upon the addition of methanol and acid. The results are displayed in Fig. 3. It can be seen that the fluorescence intensity of FCP 1 in DMF decreased gradually with increasing percentage of methanol. On the other hand, a slight increase of fluorescence intensity (4% upon 25 equiv.) was observed when FCP 1 in DMF was titrated with trifluoroacetic acid (TFA). This suggested that the increase of methanol in the solution caused stronger aggregation of the macromolecules and π-stacking interaction between neighbouring polymer chains, which in the end led to the fluorescence quenching of FCPs. On the other hand, protonation of triazole rings by TFA afforded the polymer chains to be positively charged, which kept them from aggregation and the homogeneity of the solution was enhanced. This is why a slight increase in emission intensity was observed.
1, v/v) was used as solvent to dissolve CPA to investigate the quenching efficiency of Hg2+.24 Based on these reports, it is interesting to further investigate the fluorescent behaviour of FCPs upon the addition of methanol and acid. The results are displayed in Fig. 3. It can be seen that the fluorescence intensity of FCP 1 in DMF decreased gradually with increasing percentage of methanol. On the other hand, a slight increase of fluorescence intensity (4% upon 25 equiv.) was observed when FCP 1 in DMF was titrated with trifluoroacetic acid (TFA). This suggested that the increase of methanol in the solution caused stronger aggregation of the macromolecules and π-stacking interaction between neighbouring polymer chains, which in the end led to the fluorescence quenching of FCPs. On the other hand, protonation of triazole rings by TFA afforded the polymer chains to be positively charged, which kept them from aggregation and the homogeneity of the solution was enhanced. This is why a slight increase in emission intensity was observed.
 | ||
| Fig. 3 Fluorescence intensity of FCP 1 (46 μM, corresponding to the triazole unit) in DMF upon the addition of CH3OH and TFA (inset), λex = 323 nm. | ||
The quantum yield of emission (Φf) of FCPs in DMF was 0.20, 0.24, 0.22, and 0.28 for FCP 1, 2, 3, and 4, respectively, with reference to quinine sulfate in 0.5 mol L−1 H2SO4 solution, which was calculated according to the literature method, as shown in eqn. S1.†45 The UV-vis absorption and fluorescent properties of FCPs in DMF were studied by UV-vis and fluorescence spectroscopies, respectively. The results are shown in Fig. 4. UV-vis spectra of FCPs in DMF disclosed the absorption maximum was 323 nm for FCP 1, 347 nm for FCP 2, 365 nm for FCP 3, and 340 nm for FCP 4, respectively. The analysis by fluorescence spectroscopy showed that the emission maximum of FCP 1 in DMF appeared at 373 nm with a shoulder at 359 nm under an excitation of 323 nm, while for FCP 2, 3, and 4, the emission maxima appeared at 498 nm, 489 nm, and 493 nm, respectively. Compared to the Stoke shift of FCP 1 (50 nm), FCP 2, 3 and 4 gave a much larger Stoke shift, 151 nm, 124 nm, and 153 nm, respectively. Besides, the vibrational structure observed in the emission spectrum of FCP 1 disappeared in the emission spectra of FCP 2–4.
 | ||
| Fig. 4 Absorption and fluorescence emission spectra of FCPs in DMF (con. of FCP 1, 2, 3, and 4 were 46, 41, 32, and 29 μM corresponding to the triazole unit, respectively). | ||
Considering the shoulder in the emission spectrum of FCP 1 disappeared with the addition of methanol (Fig. 3), we surmise that the shoulder corresponds to the isomers resulted from proton transfer due to the intramolecular hydrogen bonds in FCP 1, which were broken by the intermolecular hydrogen bonds formed between FCP 1 and methanol. The lack of the intramolecular hydrogen bonds in FCP 2–4, therefore no isomers formed in the solutions, could be the reason why the vibrational structure observed in the emission spectrum of FCP 1 disappeared in the emission spectra of FCP 2–4.46
The absorption maxima, emission maxima, Stoke shifts, and quantum yields of FCP 1, FCP 2, FCP 3, and FCP 4 are summarized in Table 2.
| Polymer | λabs-max | λem-max | Stoke shift (nm) | Φf |
|---|---|---|---|---|
| FCP 1 | 323 | 373 | 50 | 0.20 |
| FCP 2 | 347 | 498 | 151 | 0.24 |
| FCP 3 | 365 | 489 | 124 | 0.22 |
| FCP 4 | 340 | 493 | 153 | 0.28 |
Bearing several donor sites, 1,2,3-triazoles, known since the end of the 19th century, are now versatile ligands for metal coordination.20 FCPs synthesized via CuAAC in the present work, containing multi oxadiazole and triazole moieties, are expected to have distinctive metal ion responsive behaviors. The selectivity of FCPs for metal ions was examined by monitoring the change of fluorescence intensity upon the addition of various metal ions, including Hg2+, Cu2+, Cr3+, Cd2+, Co2+, Mn2+, Ni2+, Pb2+, Zn2+, Ba2+, Mn2+, Ca2+, K+, Na+, and Ag+. The results shown in Fig. 5 were obtained by using the solution of FCP 1 in DMF (46 μM, corresponding to the triazole unit). It was found that the fluorescence intensity of the PA changed little in the presence of most metal ions examined (10 equiv., quantity related to corresponding triazole unit). Under the same conditions, the addition of Cu2+ resulted in a moderate decrease in fluorescence intensity. In contrast, FCP 1 responded to Ag+ significantly: its fluorescence intensity decreased by 95% upon the addition of 1.0 equiv. Ag+, and the quantum yield decreased to 0.03. We also studied the kinetics of the fluorescence behaviour by adding 1.0 equiv. Ag+ into FCP 1 solution. As shown in Fig. S6,† the quenching of 90% fluorescence intensity took about 200 seconds, indicating a fast response process.
The high selectivity of FCP 1 towards Ag+ was based on the following features of Ag+ and the polytriazole: (1) the weak Lewis acidity of Ag+ endows a better affinity to the Lewis basic triazole rings;47 (2) Silver ions have a strong coordination ability to electron-rich triazole moieties in the polymer backbone,20,48 and the complexation induced the aggregation of the polymer which resulted in the fluorescence quenching.7 Thus, the combination of OXDs as fluorophores and triazole rings as metal ligands made FCP 1 an excellent fluorescent conjugate polymer sensor selectively for Ag+. The quenching mechanism (Fig. 6) is believed to relate to the quenching behavior of energy transfer along the polymer backbones12 and Ag+ induced aggregation effect.7
 | ||
| Fig. 6 The illustration of the quenching mechanism exemplified with FCP 1 in the presence of Ag+ in DMF. | ||
As is evident from Fig. 7, obvious fluorescence quenching was observed upon the increasing addition of Ag+. Coincidentally, green light from FCP 1 in DMF gradually disappeared, which was easily visible by the naked eye under UV light (λex = 365 nm).
 | ||
| Fig. 7 Fluorescence quenching of FCP 1 (46 μM, corresponding to triazole unit) in DMF upon the addition of Ag+ with increasing concentrations (0–1 equiv.), λex = 323 nm. | ||
The quenching process was analyzed by Stern–Volmer analysis according to the Stern–Volmer equation shown below:49,50
| I0/I = 1 + Ksv[Q] |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0–1
0–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 molar ratios (Fig. S7†). Based on the curve, the lowest detection limit of the polymer FCP 1 to Ag+ was decided to be 4.22 × 10−7 M, which is low enough for sensing sub-millimole concentration of Ag+ encountered practically.
1.0 molar ratios (Fig. S7†). Based on the curve, the lowest detection limit of the polymer FCP 1 to Ag+ was decided to be 4.22 × 10−7 M, which is low enough for sensing sub-millimole concentration of Ag+ encountered practically.
In addition to the prerequisites of high selectivity and sensitivity for an excellent fluorescence sensor, one more crucial requirement is minor or no disturbance from other metal ions. Thus, competition experiments were conducted by subjecting FCP 1 to appropriate mixtures of Ag+ and five folds of other various metal ions. Competition events were monitored by fluorescence spectroscopy. As shown in Fig. 8, less than 5% deviations were observed from other metals interference, which proved that the polymer could show remarkable fluorescence quenching response to Ag+ without much interference from other metal ions. On the whole, the polytriazole bridged with 2,5-diphenyl-1,3,4-oxadiazole moieties in the main chain displayed fantastic response to Ag+ with high selectivity, and sensitivity, therefore can be used as a fluorescent chemosensor for detection of Ag+.
To illustrate the potential application of FCPs, real water samples, such as tap water and water from Wutai reservoir, were used. Three concentrations of Ag+ were prepared and analysed using a DMF solution of FCP 1 (46 μM, corresponding to the triazole unit). The data were shown in Table 3 and Fig. S8.† Both water samples exhibited nice fluorescence response, the recovery yield is higher than 91% and the deviation is in the acceptable range (1.1–8.7%).
| Sample | T1 | T2 | T3 | R1 | R2 | R3 |
|---|---|---|---|---|---|---|
| C0 (10−5 M) | 1.166 | 2.000 | 2.833 | 1.166 | 2.000 | 2.833 |
| C1 (10−5 M) | 1.179 | 2.086 | 2.873 | 1.065 | 1.893 | 2.672 |
| Recovery (%) | 101.1 | 104.3 | 101.4 | 91.3 | 94.7 | 94.3 |
| SD (10−7 M) | 2.666 | 2.913 | 5.666 | 1.041 | 2.585 | 5.229 |
Optoelectronic properties of fluorescent polymers would be influenced and governed by bulky side groups tailed to the polymer backbone.7 In addition, the electronic nature of side groups changes the energy transfer of the polymer backbones, which is more relevant to the optoelectronic properties of the resultant polymers. To gain insight into the effects of side groups on the fluorescence behaviour of the polytriazoles synthesized in this work, methoxyl, n-hexyloxy, and 2-ethylhexyloxy groups tailed polymers FCP 2, FCP 3, and FCP 4 were investigated for comparison with FCP 1. The methoxyl, n-hexyloxy, and 2-ethylhexyloxy groups as not formally part of the conjugated network were expected to alter the energy transfer of the polymer backbone and minimize the aggregation of the macromolecules by interrupting the stacking of the polymer in solution, which affects the fluorescent properties of the polymers.
The selectivity and sensitivity of FCP 2, 3, and 4 were studied with similar conditions as used for FCP 1. Surprisingly, although FCP 2, 3, and 4 showed similar selectivity (Fig. S9–S11†) as FCP 1, their sensitivity for Ag+ and Cu2+ decreased considerably: the addition of 10 equiv. of Ag+ could only quench 59% fluorescence of FCP 2 (Fig. 9), 52% fluorescence of FCP 3 (Fig. 10), and 43% fluorescence of FCP 4 (Fig. 11), respectively; the addition of 10 equiv. of Cu2+ could only quench 5% fluorescence of FCP 2 (Fig. S9†), 15% fluorescence of FCP 3 (Fig. S10†), and 17% fluorescence of FCP 4 (Fig. S11†), respectively. In addition, the quenching plots of the three polymers are downward curvatures (insets in Fig. 9–11), indicating that the quenching processes involved heterogeneous population, i.e. only a fraction of triazole moieties was accessible to the quencher.54
 | ||
| Fig. 9 Fluorescence quenching of FCP 2 (41 μM, corresponding to the triazole unit) in DMF upon the addition of Ag+ with increasing concentrations (0–24 equiv.), λex = 347 nm. | ||
 | ||
| Fig. 10 Fluorescence quenching of FCP 3 (32 μM, corresponding to the triazole unit) in DMF upon the addition of Ag+ with increasing concentrations (0–55 equiv.), λex = 365 nm. | ||
 | ||
| Fig. 11 Fluorescence quenching of FCP 4 (29 μM, corresponding to the triazole unit) in DMF upon the addition of Ag+ with increasing concentrations (0–17 equiv.), λex = 340 nm. | ||
It has been proved that interchain interactions, which are less hindered by the less bulky alkoxyl side groups, promote energy transfer to lower energy chromophores,33 and the ability of metal ions to completely quench the fluorescence polymer resulted from the electron-transfer interactions between the conjugated polymer backbone and metal ion.52 Therefore, it is logical to speculate that the decreased sensitivity of FCP 2, 3, and 4 to the analytes and the difference of their quenching processes from that of FCP 1 are due to the side groups in the main chains of FCP 2 (methoxyl), FCP 3 (n-hexyloxy), and FCP 4 (2-ethylhexyloxy), which altered the polymer aggregation status7 and energy transfer in the backbone, and further influenced the fluorescence behavior of the resultant polymers.33
Conclusions
In summary, fluorescent conjugated polytriazoles (FCP 1–4) containing both 2,5-diphenyl-1,3,5-oxadiazole and 1,2,3-triazole moieties in the main chains were synthesized from aromatic diazide (1) and dialkynes (2–5) via click polymerization, respectively. In the particularly designed polymers, OXD moieties (acting as fluorophores31) and triazole rings generated via CuAAC (acting as metal ion receptors) comprise a fluorescent system. The study on their ion-responsive properties shows that, although all four FCPs have good selectivity on Ag+, the integration of alkoxy side groups as not formally part of the conjugated network to the main chains of the polytriazoles can have significant effect on the optical properties of conjugated systems, which decreased their sensitivity for Ag+ via alteration of polymer aggregation status and energy transfer of the main chains. Moreover, FCP 1 as a highly sensitive and selective fluorescent chemosensor for Ag+ was obtained via rational design, where its Ksv is as high as 1.44 × 105 M−1 and the lowest detection limit is in the ppb range (4.22 × 10−7 M). This study provides an efficient click approach to the synthesis of a novel fluorescence sensor for Ag+ detection, which could expand the application of the click polymerization in designing fluorescent sensors based on triazole units.Experimental section
Instruments and materials
All the reagents were purchased from commercial suppliers and used without further purification. THF and Et3N were purified by distillation in the presence of CaH2 before use. Other reagents and solvents were analytical grade and used as received. Flash chromatography was performed using silica gel with a grain size of 40–63 μm (Qingdao Haiyang Co., Ltd). 1H and 13C NMR spectra were recorded on a Bruker Advance 500 instrument in CDCl3, using the residual signals from CHCl3 (1H: δ 7.26 ppm; 13C: δ 77.0 ppm) as internal standard. UV-vis spectra were obtained from Shimadzu UV-1750 and fluorescence spectra were obtained from Shimadzu RF-5301. Infrared spectra were recorded on an FTIR-instrument (BRUKER TENSOR 27) with KBr pellets. Thermogravimetric analysis (TGA) thermograms were recorded on an auto-simultaneous instrument of thermogravimetry and differential thermal analysis (Shimazu DTG-60A). Differential Scanning Calorimetry analysis (DSC) thermograms were recorded on a TA DSC Q2000 instrument. Molecular weight was determined by gel permeation chromatography (GPC) with Waters-515 HPLC pump using DMF as solvent and compared to polystyrene standards. Metal ions such as nitrate salts or perchlorate salts were used to prepare metal ion stock solutions. 2,5-Bis(p-aminophenyl)-1,3,4-oxadiazole (6), 1,4-di(2-methyl-2-hydroxy-3-butynyl)-benzene (7), 1,4-di(2-methyl-2-hydroxy-3-butynyl)-benzene (8), 1,4-bis(2-methylbut-3-yn-2-ol)-2,5-bis(hexyloxy)benzene (11a), 1,4-bis(trimethylsilylethynyl)-2,5-bis(2-ethylhexyloxy)benzene (11b) were prepared according to the publish procedures32,33,36,37,55 (details can be found in ESI†).Synthesis of monomer 1–5 and FCPs
Synthesis of FCPs via copper-catalyzed click polymerization
To a 5 mL flask were added 1 (0.1 mmol), 2 (0.1 mmol), and Cu(PPh3)3Br (0.005 mmol) under nitrogen. The mixture was degassed by several cycles. Then dry DMF (1.5 mL) was injected into the flask to dissolve the monomers and catalyst. The reaction mixture was degassed one more time and the reaction flask was evacuated and flushed with argon. The reaction mixture was stirred at 50 °C for 12 h. Then, the mixture was diluted with chloroform and added drop wise to a mixture of a hexane/chloroform (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume). The precipitate was allowed to stand overnight, then collected and dried to get FCP 1 with a yield of 37% (Scheme 2e). By replacing monomer 2 with 3, 4, or 5, FCP 2, FCP 3, and FCP 4 were synthesized by following the same procedure with a yield of 49%, 61%, or 67%, respectively. FTIR (KBr) ν (cm−1): FCP 1 3296, 3106, 2127, 2092, 1610, 1508, 1490; FCP 2 3292, 3096, 2940, 2840, 2120, 2091, 1603, 1504, 1495; FCP 3 3310, 3061, 2924, 2854, 2125, 2091, 1671, 1502, 1492; FCP 4 3309, 3059, 2954, 2922, 2855, 2129, 2091, 1611, 1502, 1499.
1 by volume). The precipitate was allowed to stand overnight, then collected and dried to get FCP 1 with a yield of 37% (Scheme 2e). By replacing monomer 2 with 3, 4, or 5, FCP 2, FCP 3, and FCP 4 were synthesized by following the same procedure with a yield of 49%, 61%, or 67%, respectively. FTIR (KBr) ν (cm−1): FCP 1 3296, 3106, 2127, 2092, 1610, 1508, 1490; FCP 2 3292, 3096, 2940, 2840, 2120, 2091, 1603, 1504, 1495; FCP 3 3310, 3061, 2924, 2854, 2125, 2091, 1671, 1502, 1492; FCP 4 3309, 3059, 2954, 2922, 2855, 2129, 2091, 1611, 1502, 1499.
Acknowledgements
We thank the National Natural Science Foundation of China for financial support (NSFC21174113). The authors also thank Mr. Yihan Pei from Clare College, University of Cambridge for the help with language.Notes and references
- H. T. Ratte, Environ. Toxicol. Chem., 1999, 18, 89–108 CrossRef CAS PubMed.
- D. S. Merrell and A. Camilli, J. Bacteriol., 2000, 182, 5342–5350 CrossRef CAS.
- R. Behra, L. Sigg, M. J. Clift, F. Herzog, M. Minghetti, B. Johnston, A. Petri-Fink and B. Rothen-Rutishauser, J. R. Soc., Interface, 2013, 10, 20130396 CrossRef PubMed.
- L. Liu, G. Zhang, J. Xiang, D. Zhang and D. Zhu, Org. Lett., 2008, 10, 4581–4584 CrossRef CAS PubMed.
- M. Kumar, R. Kumar and V. Bhalla, Org. Lett., 2011, 13, 366–369 CrossRef CAS PubMed.
- N. J. Wang, C. M. Sun and W. S. Chung, Sens. Actuators, B, 2012, 171–172, 984–993 CrossRef CAS PubMed.
- H. Tong, L. X. Wang, X. B. Jing and F. S. Wang, Macromolecules, 2002, 35, 7169–7171 CrossRef CAS.
- C. Qin, W.-Y. Wong and L. Wang, Macromolecules, 2011, 44, 483–489 CrossRef CAS.
- Y. Bao, Q. Li, B. Liu, F. Du, J. Tian, H. Wang, Y. Wang and R. Bai, Chem. Commun., 2012, 48, 118–120 RSC.
- S. Sun, B. Qiao, N. Jiang, J. Wang, S. Zhang and X. Peng, Org. Lett., 2014, 16, 1132–1135 CrossRef CAS PubMed.
- Y. J. Gong, X. B. Zhang, C. C. Zhang, A. L. Luo, T. Fu, W. h. Tan, G. L. Shen and R. Q. Yu, Anal. Chem., 2012, 84, 10777–10784 CrossRef CAS PubMed.
- C. B. Murphy, Y. Zhang, T. Troxler, V. Ferry, J. J. Martin and W. E. Jones, J. Phys. Chem. B, 2004, 108, 1537–1543 CrossRef CAS.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS.
- E. Zhao, H. Li, J. Ling, H. Wu, J. Wang, S. Zhang, J. W. Lam, J. Z. Sun, A. Qin and B. Z. Tang, Polym. Chem., 2014, 5, 2301–2308 RSC.
- W. Z. Yuan, Z.-Q. Yu, Y. Tang, J. W. Y. Lam, N. Xie, P. Lu, E.-Q Chen, Qiang and B. Z. Tang, Macromolecules, 2011, 44, 9618–9628 CrossRef CAS.
- S. Bakbak, P. J. Leech, B. E. Carson, S. Saxena, W. P. King and U. H. F. Bunz, Macromolecules, 2006, 39, 6793–6795 CrossRef CAS.
- O. Plietzsch, C. I. Schilling, T. Grab, S. L. Grage, A. S. Ulrich, A. Comotti, P. Sozzani, T. Muller and S. Braese, New J. Chem., 2011, 35, 1577–1581 RSC.
- A. Qin, J. W. Y. Lam, L. Tang, C. K. W. Jim, H. Zhao, J. Sun and B. Z. Tang, Macromolecules, 2009, 42, 1421–1424 CrossRef CAS.
- G. Nagarjuna, A. Kumar, A. Kokil, K. G. Jadhav, S. Yurt, J. Kumar and D. Venkataraman, J. Mater. Chem., 2011, 21, 16597 RSC.
- J. J. Bryant and U. H. Bunz, Chem.–Asian J., 2013, 8, 1354–1367 CrossRef CAS PubMed.
- S. W. Thomas, G. D. Joly and T. M. Swager, Chem. Rev., 2007, 107, 1339–1386 CrossRef CAS PubMed.
- X. Huang, J. Meng, Y. Dong, Y. Cheng and C. Zhu, Polymer, 2010, 51, 3064–3067 CrossRef CAS PubMed.
- Y. Cheng, L. Zheng, X. Huang and Y. Shen, Synlett, 2010, 3, 453–456 CrossRef PubMed.
- Y. Wu, Y. Dong, J. Li, X. Huang, Y. Cheng and C. Zhu, Chem.–Asian J., 2011, 6, 2725–2729 CrossRef CAS PubMed.
- C. Zhu, Y. Cheng, X. Huang, Y. Dong and J. Meng, Synlett, 2010, 12, 1841–1844 Search PubMed.
- Y. T. Chang, J. K. Chang, Y. T. Lee, P. S. Wang, J. L. Wu, C. C. Hsu, I. W. Wu, W. H. Tseng, T. W. Pi, C. T. Chen and C. I. Wu, ACS Appl. Mater. Interfaces, 2013, 5, 10614–10622 CAS.
- T. Goudreault, Z. He, Y. Guo, C.-L. Ho, H. Zhan, Q. Wang, K. Y.-F. Ho, K.-L. Wong, D. Fortin, B. Yao, Z. Xie, L. Wang, W.-M. Kwok, P. D. Harvey and W.-Y. Wong, Macromolecules, 2010, 43, 7936–7949 CrossRef CAS.
- T. Higashihara, H. Wu, T. Mizobe, C. Lu, M. Ueda and W. Chen, Macromolecules, 2012, 45, 9046–9055 CrossRef CAS.
- C. S. Wang, L. O. Palsson, A. S. Batsanov and M. R. Bryce, J. Am. Chem. Soc., 2006, 128, 3789–3799 CrossRef CAS PubMed.
- Y. Liu, L. Zong, L. Zheng, L. Wu and Y. Cheng, Polymer, 2007, 48, 6799–6807 CrossRef CAS PubMed.
- P. H. Aubert, M. Knipper, L. Groenendaal, L. Lutsen, J. Manca and D. Vanderzande, Macromolecules, 2004, 37, 4087–4098 CrossRef CAS.
- J. R. Thomas, X. J. Liu and P. J. Hergenrother, J. Am. Chem. Soc., 2005, 127, 12434–12435 CrossRef CAS PubMed.
- M. C. Baier, J. Huber and S. Mecking, J. Am. Chem. Soc., 2009, 131, 14267–14273 CrossRef CAS PubMed.
- R. E. Palacios, W. S. Chang, J. K. Grey, Y. L. Chang, W. L. Miller, C. Y. Lu, G. Henkelman, D. Zepeda, J. Ferraris and P. F. Barbara, J. Phys. Chem. B, 2009, 113, 14619–14628 CrossRef CAS PubMed.
- L. Vacareanu, I. M. Dorin and M. Grigoras, Rev. Chim., 2010, 61, 74–77 CAS.
- B. Liu, Y. Bao, F. Du, H. Wang, J. Tian and R. Bai, Chem. Commun., 2011, 47, 1731–1733 RSC.
- W. Shu, C. Guan, W. Guo, C. Wang and Y. Shen, J. Mater. Chem., 2012, 22, 3075–3081 RSC.
- Y. Hou, S. Cao, L. Wang, Y. Pei, G. Zhang, S. Zhang and Z. Pei, Polym. Chem., 2015, 6, 223–227 RSC.
- V. Y. Grinshtein, A. A. Strazdin and A. K. Grinvalde, Chem. Heterocycl. Compd., 1970, 6, 231–239 CrossRef.
- A. A. Zahraa and J. J. Amer, J. Al-Qadisiyah Pure Sci., 2011, 16, 1–17 Search PubMed.
- Z. Khosrow, F. Khalil, S. Reza and Z. Javad, Turk. J. Chem., 2003, 27, 119–125 Search PubMed.
- A. Faeza and H. Nazik, J. Basrah Res., 2014, 40, 146–159 Search PubMed.
- M. S. Al-Ajely, H. M. Al-Ajely and A. N. Al-NaibH, Tikrit J. Pure Sci., 2008, 13, 100–106 Search PubMed.
- J. Herbich, C. Y. Hung, R. P. Thummel and J. Waluk, J. Am. Chem. Soc., 1996, 118, 3508–3518 CrossRef CAS.
- D. F. Eaton, Pure Appl. Chem., 1988, 60, 1107–1114 CrossRef CAS.
- Y. Y. Zhu, G. T. Wang, R. X. Wang and Z. T. Li, Cryst. Growth Des., 2009, 9, 4778–4783 CAS.
- R. A. Bell and J. R. Kramer, Environ. Toxicol. Chem., 1999, 18, 9–22 CAS.
- Z. Zhou and C. J. Fahrni, J. Am. Chem. Soc., 2004, 126, 8862–8863 CrossRef CAS PubMed.
- B. Valeur, Molecular Fluorescence: Principles and Applications, Wiley-VCH, Weinheim, Germany, 2002 Search PubMed.
- E. Blatt, A. Mau, W. Sasse and W. Sawyer, Aust. J. Chem., 1988, 41, 127–131 CrossRef CAS.
- J. Lakowicz, Principals of Fluorescence Spectroscopy, Plenum Press, New York, 1983 Search PubMed.
- V. Banjoko, Y. Xu, E. Mintz and Y. Pang, Polymer, 2009, 50, 2001–2009 CrossRef CAS PubMed.
- L. Q. Ying and B. P. Branchaud, Chem. Commun., 2011, 47, 8593–8595 RSC.
- J. Lakowicz, Principles of Fluorescence Spectroscopy, Plenum Publishing Corporation, New York, 2nd edn, 1999 Search PubMed.
- Z. Li, L. Liu, W. Zhang, C. Sue, Q. Li, O. S. Miljanic, O. M. Yaghi and J. F. Stoddart, Chem–Eur. J., 2009, 15, 13356–13380 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and additional results including fluorescence curves, NMR data and FTIR spectra. See DOI: 10.1039/c5ra07822a |
| This journal is © The Royal Society of Chemistry 2015 |