Non-covalent functionalization of carbon nano-onions with pyrene–BODIPY dyads for biological imaging†
Abstract
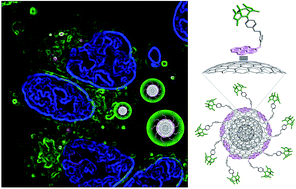
We report a novel approach based on non-covalent interactions for the functionalization of carbon nano-onions (CNOs) with fluorophores. The assembly of pyrene–BODIPY conjugates on the CNO surface by means of π–π-stacking results in fluorescent carbon nanoparticles that are successfully uptaken by HeLa cancer cells with no cytotoxicity observed. The ability to functionalize carbon-based nanomaterials by using mild conditions will pave the way for future clinical application of these versatile nanomaterials.

 Please wait while we load your content...
Please wait while we load your content...