A conjugated ketone as a catalyst in alcohol amination reactions under transition-metal and hetero-atom free conditions†
Xingchao Dai,
Xinjiang Cui,
Youquan Deng and
Feng Shi*
State Key Laboratory for Oxo Synthesis and Selective Oxidation, Centre for Green Chemistry and Catalysis, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou, 730000, China. E-mail: fshi@licp.cas.cn; Fax: +86-931-8277088; Tel: +86-931-4968142
First published on 30th April 2015
Abstract
Here, we show the results of a molecular-defined conjugated ketone catalyzed alcohol amination reaction. Under the optimized reaction conditions, the yields to the desired products reached 98%. The reaction mechanism and kinetic study supposed that carbonyl–hydroxyl groups are the catalytically active sites, and the transfer-hydrogenation reactions progress via the recycling of carbonyl and hydroxyl groups. The catalytic process shows promise as an efficient and economic route for alcohol amination reactions.
Alcohols are some of the best established reactants in chemistry. Normally the catalytic activation of an alcohol is realized with two different mechanisms.1 The first one is the activation of the alcohol with the addition of an acid or base as a catalyst to generate electrophilic or nucleophilic species. Alternatively, the alcohol can be activated in the presence of a dehydrogenative catalyst. In this way, a carbonyl compound will be generated. The carbonyl group can further react with other reactants, typically, amine derivatives, to form imines via dehydration. Ideally the hydride adsorbed onto the catalyst can be transferred to an imine again and an N-alkyl amine can be synthesized. In the whole process, the sole byproduct is water and the whole process is processed via a transfer-hydrogenation reaction mechanism.
In order to realize these transformations, the presence of transition-metal catalysts is normally essential. In the alcohol amination reactions, a series of homogeneous or heterogeneous transition-metal catalysts such as ruthenium,2 iridium,3 palladium,4 rhodium,5 platinum,6 gold,7 Re,8 silver,9 nickel,10 manganese,11 copper12 and iron13 are developed and excellent results are obtained. Recently, boron, aldehyde and carbon based catalysts have been shown to be active catalysts in the transfer-hydrogenation reactions. For example, the catalytic reduction of imine, nitrile, olefin, carbon dioxide and other compounds has been reported using boron complexes as catalysts via the formation of borohydride as the intermediate.14 Aldehyde molecules have been shown active catalysts in alcohol amination reactions.15 In addition, carbon material can be perfect catalyst in the catalytic reduction of nitro compounds with hydrazine as the reducing agent, and in the alcohol amination reactions.16
Based on the extensive results of the transfer hydrogenation reactions and alcohol amination reactions, it can be concluded that the key steps are the dehydrogenation of alcohol to generate carbonyl group and the re-addition of the hydride to the unsaturated imine bond. In fact it is the recycling of hydride between carbonyl and hydroxyl groups. It inspires us that possibly we can perform the transfer-hydrogenation reaction with ketone as the catalyst. The versatile structure of ketone derivatives gives us great opportunity to tune the catalytic activity. After screening a series of ketone derivatives with different structures, the alcohol amination reactions are realized with high efficiency catalyzed by a simple xanthen-9-one organocatalyst (Scheme 1). N-Alkyl amines and alcohols can be synthesized with up to 98% isolated yields via alcohol amination. Reaction mechanism and kinetic study prove the recycling of ketone and hydroxyl groups during the catalytic reaction, i.e. the hydride is first transferred from alcohol to the ketone group of the catalyst, then the hydride is transferred to the desired product and the ketone group is re-generated. The detailed results and discussions are given below.
The catalytic activity of different ketones in transfer-hydrogenation reactions was explored with aniline and benzyl alcohol as starting materials to synthesize N-benzyl aniline (Table 1). As the typical base and solvent, KOH and toluene were employed. Clearly catalysts cat-1 to cat-6 exhibited nice catalytic performance (entries 1–7). By applying cat-1 as catalyst, the conversion of aniline was 63% and the yield of N-benzyl aniline was 35%. If cat-2 was used as the catalyst, the conversion of aniline and the selectivity to N-benzyl aniline can be improved significantly, which resulted in 94% aniline conversion and 80% N-benzyl aniline yield. The incorporation of substituents, such as methoxyl, dimethylamino and diethylamino groups, onto the aromatic ring influences the reactivity remarkably. The conversions of aniline were 47–55% with 31–44% N-benzyl aniline yields. If cat-6 was used as catalyst, it can be seen that 54% aniline conversion and 49% N-benzyl aniline yield were obtained but almost no reaction occurred if cat-7 was applied. Relatively good results were also obtained if cat-8 was employed, which conversion was 95% and the yield of N-benzyl aniline was 80% (entry 8). Noteworthy excellent results were obtained if cat-9 was used as the catalyst, which conversion was 99% with 96% N-benzyl aniline yield (entry 9). Then poor results were obtained if cat-10 and cat-11 were used as catalysts (entries 10–11), which mean that the formation of fused ring compound is unfavorable to the transfer-hydrogenation reactions. Following, the influence of base was studied (entries 12–16). Clearly, the applying of strong base is helpful for the transfer-hydrogenation reactions. Lower conversion and selectivity were obtained if NaOH, K2CO3, Na2CO3 and CaO were employed. Poor results were obtained if the reaction was performed under base free conditions, the conversion of aniline was <1%. Then the solvent variation was explored. Commonly organic solvents including xylene, mesitylene, dioxane, acetonitrile, tetrahydrofuran and octane were applied in the transfer-hydrogenation reactions (entries 17–22). Clearly, the properties of the solvents influenced the transfer-hydrogenation reaction remarkably and nonpolar solvents are helpful to the reaction. It should be mentioned that although 99% aniline conversion was achieved when tetrahydrofuran was used as solvent almost no desired product was obtained. The major products were 1-phenylpyrrolidine and 1-phenyl-pyrrole. In addition, relatively good results were obtained if 1.2 equiv., of benzyl alcohol was employed (entries 23). Only 11% desired product was obtained if KOH itself was used as catalyst (entry 24). Therefore, the ketone should be the main catalyst.
| Entry | Catal. | Bases | Solvents | Conv.b/% | Y.b/% |
|---|---|---|---|---|---|
| a Reaction conditions: 1 mmol aniline, 1.5 mmol benzyl alcohol, 50 mg catalyst, 0.5 mmol KOH, 2 mL solvent, Ar, 150 °C, 4 h.b Conversion of and yield determined by GC-FID with biphenyl as an external standard material.c Isolated yield.d The products were 1-phenylpyrrolidine and 1-phenyl-pyrrole.e 1.2 mmol benzyl alcohol was used. | |||||
| 1 | cat-1 | KOH | PhMe | 63 | 35 |
| 2 | cat-2 | KOH | PhMe | 94 | 80 |
| 3 | cat-3 | KOH | PhMe | 53 | 31 |
| 4 | cat-4 | KOH | PhMe | 47 | 33 |
| 5 | cat-5 | KOH | PhMe | 55 | 44 |
| 6 | cat-6 | KOH | PhMe | 54 | 49 |
| 7 | cat-7 | KOH | PhMe | 4 | <1 |
| 8 | cat-8 | KOH | PhMe | 95 | 80 |
| 9 | cat-9 | KOH | PhMe | 99 | 96 (95c) |
| 10 | cat-10 | KOH | PhMe | <1 | — |
| 11 | cat-11 | KOH | PhMe | <1 | — |
| 12 | cat-9 | NaOH | PhMe | 33 | 19 |
| 13 | cat-9 | K2CO3 | PhMe | <1 | — |
| 14 | cat-9 | Na2CO3 | PhMe | <1 | — |
| 15 | cat-9 | CaO | PhMe | 8 | <1 |
| 16 | cat-9 | — | PhMe | <1 | — |
| 17 | cat-9 | KOH | Xylene | 59 | 50 |
| 18 | cat-9 | KOH | Mesitylene | 22 | 15 |
| 19 | cat-9 | KOH | Dioxane | 7 | 5 |
| 20 | cat-9 | KOH | CH3CN | 7 | <1 |
| 21 | cat-9 | KOH | THF | 99 | <1d |
| 22 | cat-9 | KOH | Octane | 35 | 27 |
| 23e | cat-9 | KOH | PhMe | 90 | 85 |
| 24 | — | KOH | PhMe | 13 | 11 |
Next, this optimized reaction condition was applied in the alcohol amination reactions with different amine and alcohol using cat-9 as catalyst (Table 2). Various structurally diverse amines, regardless of the presence of electron-withdrawing or electron-donating functional groups, were monoalkylated with benzyl alcohol to yield the corresponding secondary amines in excellent yields. For example, N-benzyl aniline was synthesized in 95% yield in 4 h (entry 1). The substituents at different positions on the aniline significantly affected the reaction rate. For example, a lower yield was obtained when o-methylaniline was used as a substrate compared with p-methylaniline and m-methylaniline (entries 2–4). Excellent results were also obtained if other alkyl substituents, i.e., ethyl, butyl, tert-butyl, hexyl and di-methyl groups, were involved (entries 5–10). The yields of the desired products were 84–98%. The reaction tolerated the presence of chloro and methoxyl groups (entries 11–13), and 80–98% yields of the desired products were observed when m- or p-chloro or m-MeO-anilines were used as starting materials. The reactions of 2-aminopyridine, naphthalen-1-amine and benzidine with benzyl alcohol resulted in 95–98% yields (entries 14–16). The reaction also proceeded successfully with other structurally and electronically diverse alcohols. Benzyl alcohol with moderately activating groups reacted smoothly and furnished excellent yields of the respective mono-N-alkylated anilines (entries 17–21). Alcohols with a chlorine or fluoro substituent reacted with aniline to afford the corresponding products with 100% conversion with up to 95% yields (entries 22–23). The cat-9 catalyst also exhibits promise for direct coupling of aniline with simple heteroaromatic alcohols under similar reaction conditions in excellent yields (entries 24–25). p-Methylthiobenzyl alcohol was converted to the respective secondary amine in 95% yield (entry 26). To our delight, aliphatic primary amine and aliphatic primary alcohol, i.e., butyl amine, butanol and octanol can also be used in the ketone molecule catalyzed transfer-hydrogenation reactions. Typically the starting materials can be converted efficiently but a mixture of the N-alkyl amine and N-alkyl imine products were obtained (entries 27–29). These results suggest a nice generality of the molecular-defined ketone catalyst in the alcohol amination reactions with different amine and alcohols as starting materials.
| Entry | R1 | R2 | Product | t/h | Y.b/% |
|---|---|---|---|---|---|
| a Reaction conditions: 1 mmol amine, 1.5 mmol alcohol, 50 mg cat-9, 0.5 mmol KOH, 2 mL toluene, Ar, 150 °C, 4–24 h.b Isolated yield.c Conversions of the amines determined by GC-MS.d Selectivities of N-alkyl amine and N-alkyl imine determined by GC-MS. | |||||
| 1 | Ph | Ph |  |
4 | 95 |
| 2 | o-Me-Ph | Ph |  |
8 | 61 |
| 3 | m-Me-Ph | Ph |  |
4 | 97 |
| 4 | p-Me-Ph | Ph |  |
4 | 93 |
| 5 | m-Et-Ph | Ph |  |
8 | 84 |
| 6 | p-Bu-Ph | Ph |  |
8 | 85 |
| 7 | p-tert-Bu-Ph | Ph- |  |
8 | 92 |
| 8 | p-Hexyl-Ph | Ph | 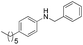 |
8 | 88 |
| 9 | 3,4-Di-Me-Ph | Ph |  |
8 | 98 |
| 10 | 3,5-Di-Me-Ph | Ph |  |
8 | 86 |
| 11 | m-Cl-Ph | Ph |  |
8 | 98 |
| 12 | p-Cl-Ph | Ph |  |
4 | 92 |
| 13 | p-MeO-Ph | Ph |  |
4 | 80 |
| 14 | 2-Py | Ph |  |
8 | 98 |
| 15 | 1-Naphthyl | Ph |  |
4 | 95 |
| 16 | Benzidine | Ph |  |
8 | 87 |
| 17 | Ph | o-Me-Ph |  |
4 | 96 |
| 18 | Ph | m-Me-Ph |  |
4 | 96 |
| 19 | Ph | p-Me-Ph |  |
4 | 84 |
| 20 | Ph | o-MeO-Ph | 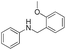 |
8 | 83 |
| 21 | Ph | m-PhO-Ph |  |
8 | 97 |
| 22 | Ph | m-Cl-Ph |  |
8 | 95 |
| 23 | Ph | m-F-Ph |  |
8 | 64 |
| 24 | Ph | 2-Py |  |
8 | 98 |
| 25 | Ph | 3-Py |  |
8 | 76 |
| 26 | Ph | p-MeS-Ph |  |
8 | 95 |
| 27 | Ph | Butyl |  |
24 | 53c(65/15d) |
| 28 | Bu- | Ph- |  |
24 | 77c(20/73d) |
| 29 | Bu- | Octyl- |  |
24 | 78c(0/99d) |
In order to explain the catalytic activity variation of different catalysts, the transformations of the catalysts during catalytic reaction were explored with cat-2, cat-3 and cat-9 as the model catalysts. By analyzing the reaction mixtures after reactions (Table 1), it can be seen that a part of the catalysts were transferred to the corresponding alcohols. These results showed that catalytic cycle might be between carbonyl and hydroxyl groups, and the hydrogenation ability of the ketone and dehydrogenation ability of the corresponding alcohol should be responsible for the catalytic activity variation. In order to clarify the reaction mechanism and catalytic activity variation of different catalysts, following control reactions were performed under the given reaction conditions in Table 1, i.e., (a) reaction of cat-2 with benzyl alcohol, (b) reaction of cat-3 with benzyl alcohol, (c) reaction of cat-9 with benzyl alcohol, (d) reaction of cat-2-OH with benzaldehyde/aniline, (e) reaction of cat-3-OH with benzaldehyde/aniline and (f) reaction of cat-9-OH with benzaldehyde/aniline (Scheme 2). After reaction, a mixture of ketones, i.e., cat-2, 3 and 9, and the corresponding alcohols were formed. The ratios of ketones to the alcohols were 7.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 92.5, 28
92.5, 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 72 and 98
72 and 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in reactions (a) to (c). The results suggested that cat-2 could be easily hydrogenated to cat-2-OH but cat-9 was different to be reduced under the same reaction conditions. About cat-3, it can be smoothly reduced to cat-3-OH, too. During these transformations, benzyl alcohol was oxidized to benzaldehyde. Interestingly, the ratios of the ketones to the corresponding alcohols were 41
2 in reactions (a) to (c). The results suggested that cat-2 could be easily hydrogenated to cat-2-OH but cat-9 was different to be reduced under the same reaction conditions. About cat-3, it can be smoothly reduced to cat-3-OH, too. During these transformations, benzyl alcohol was oxidized to benzaldehyde. Interestingly, the ratios of the ketones to the corresponding alcohols were 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 59, ≪1
59, ≪1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 and ≫100
99 and ≫100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in reactions (d) to (f). These results showed that the hydride can be efficiently transferred from cat-9-OH to the imine intermediate (benzaldehyde + aniline) to generate N-benzyl aniline while the dehydrogenation of cat-3-OH was quite difficult. About cat-2-OH, its dehydrogenation ability was moderate. It should be interesting if taking the results of N-alkyl aniline synthesis with cat-2, 3 and 9 as catalysts into consideration together. The conversions of aniline were 94%, 53% and 99% with 85%, 58% and 97% N-benzyl aniline selectivities (Table 1, entries 2, 3 and 9). The relatively good results with cat-2 as catalyst might be attributed to the good recycling between cat-2 and cat-2-OH. For the excellent catalytic activity of cat-9, it can be explained by the extremely good performance of the transferring of hydrogen atom from cat-9-OH to the imine intermediate so it can promote the progress of the reaction efficiently. About catalyst cat-3, although it is active for the activation of benzyl alcohol to generate benzaldehyde, it is less active for the transfer-hydrogenation of imine intermediate from cat-3-OH to form the desired product, which might be responsible for the lower activity. Based on the above discussion, it can be concluded partially that the crucial step in the catalyst recycling might be the transferring of hydride to the imine intermediate from cat-OH and a reaction mechanism was proposed as Scheme 2(g).
1 in reactions (d) to (f). These results showed that the hydride can be efficiently transferred from cat-9-OH to the imine intermediate (benzaldehyde + aniline) to generate N-benzyl aniline while the dehydrogenation of cat-3-OH was quite difficult. About cat-2-OH, its dehydrogenation ability was moderate. It should be interesting if taking the results of N-alkyl aniline synthesis with cat-2, 3 and 9 as catalysts into consideration together. The conversions of aniline were 94%, 53% and 99% with 85%, 58% and 97% N-benzyl aniline selectivities (Table 1, entries 2, 3 and 9). The relatively good results with cat-2 as catalyst might be attributed to the good recycling between cat-2 and cat-2-OH. For the excellent catalytic activity of cat-9, it can be explained by the extremely good performance of the transferring of hydrogen atom from cat-9-OH to the imine intermediate so it can promote the progress of the reaction efficiently. About catalyst cat-3, although it is active for the activation of benzyl alcohol to generate benzaldehyde, it is less active for the transfer-hydrogenation of imine intermediate from cat-3-OH to form the desired product, which might be responsible for the lower activity. Based on the above discussion, it can be concluded partially that the crucial step in the catalyst recycling might be the transferring of hydride to the imine intermediate from cat-OH and a reaction mechanism was proposed as Scheme 2(g).
 | ||
| Scheme 2 Reaction mechanism exploration. The results were determined by GC-MS with external standard method. | ||
In conclusion, an efficient molecular-defined organocatalyst was developed for the transfer-hydrogenation reactions including alcohol amination and carbonyl compound reduction. The ketone and alcohol groups inside the catalyst are the active sites of the catalyst. To the best of our knowledge, this is the first time to show that ketone with a specific structure can be an active catalyst for transfer-hydrogenation reactions. It should be helpful for us to understand the transfer-hydrogenation reaction and also provides an efficient methodology for such transformations.
Notes and references
- (a) M. H. S. A. Hamid, P. A. Slatford and J. M. J. Williams, Adv. Synth. Catal., 2007, 349, 1555–1575 CrossRef CAS PubMed; (b) G. Guillena, D. J. Ramon and M. Yus, Chem. Rev., 2010, 110, 1611–1641 CrossRef CAS PubMed; (c) G. E. Dobereiner and R. H. Crabtree, Chem. Rev., 2010, 110, 681–703 CrossRef CAS PubMed; (d) S. Bähn, S. Imm, L. Neubert, M. Zhang, H. Neumann and M. Beller, ChemCatChem, 2011, 3, 1853–1864 CrossRef PubMed.
- (a) S. Imm, S. Bahn, M. Zhang, L. Neubert, H. Neumann, F. Klasovsky, J. Pfeffer, T. Haas and M. Beller, Angew. Chem., Int. Ed., 2011, 50, 7599–7603 CrossRef CAS PubMed; (b) A. J. Watson, A. C. Maxwell and J. M. Williams, J. Org. Chem., 2011, 76, 2328–2331 CrossRef CAS PubMed; (c) K. Yamaguchi, J. He, T. Oishi and N. Mizuno, Chem.–Eur. J., 2010, 16, 7199–7207 CrossRef CAS PubMed; (d) X. Ye, P. N. Plessow, M. K. Brinks, M. Schelwies, T. Schaub, F. Rominger, R. Paciello, M. Limbach and P. Hofmann, J. Am. Chem. Soc., 2014, 136, 5923–5929 CrossRef CAS PubMed.
- (a) K. Rafikova, N. Kystaubayeva, M. Aydemir, C. Kayan, Y. S. Ocak, H. Temel, A. Zazybin, N. Gurbuz and I. Ozdemir, J. Organomet. Chem., 2014, 758, 1–8 CrossRef CAS PubMed; (b) J. Norinder and A. Börner, ChemCatChem, 2011, 3, 1407–1409 CrossRef CAS PubMed; (c) Y. Zhang, C. S. Lim, D. S. Sim, H. J. Pan and Y. Zhao, Angew. Chem., Int. Ed., 2014, 53, 1399–1403 CrossRef CAS PubMed; (d) F. Li, H. Shan, L. Chen, Q. Kang and P. Zou, Chem. Commun., 2012, 48, 603–605 RSC; (e) H. Ohta, Y. Yuyama, Y. Uozumi and Y. M. A. Yamada, Org. Lett., 2011, 13, 3892–3895 CrossRef CAS PubMed; (f) O. Saidi, A. J. Blacker, M. M. Farah, S. P. Marsden and J. M. Williams, Angew. Chem., Int. Ed., 2009, 48, 7375–7378 CrossRef CAS PubMed.
- (a) M. Ousmane, G. Perrussel, Z. Yan, J. M. Clacens, F. De Campo and M. Pera-Titus, J. Catal., 2014, 309, 439–452 CrossRef CAS PubMed; (b) Y. Zhang, X. Qi, X. Cui, F. Shi and Y. Deng, Tetrahedron Lett., 2011, 52, 1334–1338 CrossRef CAS PubMed; (c) R. Ghosh and A. Sarkar, J. Org. Chem., 2011, 76, 8508–8512 CrossRef CAS PubMed.
- S. L. Feng, C. Z. Liu, Q. Li, X. C. Yu and Q. Xu, Chin. Chem. Lett., 2011, 22, 1021–1024 CrossRef CAS PubMed.
- (a) W. He, L. Wang, C. Sun, K. Wu, S. He, J. Chen, P. Wu and Z. Yu, Chem.–Eur. J., 2011, 17, 13308–13317 CrossRef CAS PubMed; (b) L. Wang, W. He, K. Wu, S. He, C. Sun and Z. Yu, Tetrahedron Lett., 2011, 52, 7103–7107 CrossRef CAS PubMed.
- (a) Y. S. Demidova, I. L. Simakova, J. Wärnå, A. Simakov and D. Y. Murzin, Chem. Eng. J., 2014, 238, 164–171 CrossRef CAS PubMed; (b) Y. Wang, D. Zhu, L. Tang, S. Wang and Z. Wang, Angew. Chem., Int. Ed., 2011, 50, 8917–8921 CrossRef CAS PubMed; (c) L. He, X. B. Lou, J. Ni, Y. M. Liu, Y. Cao, H. Y. He and K. N. Fan, Chem.–Eur. J., 2010, 16, 13965–13969 CrossRef CAS PubMed.
- A. Abdukader, H. Jin, Y. Cheng and C. Zhu, Tetrahedron Lett., 2014, 55, 4172–4174 CrossRef CAS PubMed.
- (a) I. Geukens, F. Vermoortele, M. Meledina, S. Turner, G. Van Tendeloo and D. E. De Vos, Appl. Catal., A, 2014, 469, 373–379 CrossRef CAS PubMed; (b) X. Cui, Y. Zhang, F. Shi and Y. Deng, Chem.–Eur. J., 2011, 17, 1021–1028 CrossRef CAS PubMed; (c) K.-I. Shimizu, K. Shimura, M. Nishimura and A. Satsuma, RSC Adv., 2011, 1, 1310–1317 RSC.
- (a) K.-i. Shimizu, K. Kon, W. Onodera, H. Yamazaki and J. N. Kondo, ACS Catal., 2013, 3, 112–117 CrossRef CAS; (b) K.-i. Shimizu, N. Imaiida, K. Kon, S. M. A. Hakim Siddiki and A. Satsuma, ACS Catal., 2013, 3, 998–1005 CrossRef CAS.
- X. Yu, C. Liu, L. Jiang and Q. Xu, Org. Lett., 2011, 13, 6184–6187 CrossRef CAS PubMed.
- (a) X. Chen, H. Luo, C. Qian and C. He, React. Kinet., Mech. Catal., 2011, 104, 163–171 CrossRef CAS; (b) X. Cui, X. Dai, Y. Deng and F. Shi, Chem.–Eur. J., 2013, 19, 3665–3675 CrossRef CAS PubMed; (c) A. Martínez-Asencio, D. J. Ramón and M. Yus, Tetrahedron Lett., 2010, 51, 325–327 CrossRef PubMed; (d) J. He, K. Yamaguchi and N. Mizuno, Chem. Lett., 2010, 39, 1182–1183 CrossRef CAS.
- (a) Y. Zhao, S. W. Foo and S. Saito, Angew. Chem., Int. Ed., 2011, 50, 3006–3009 CrossRef CAS PubMed; (b) X. Cui, F. Shi, Y. Zhang and Y. Deng, Tetrahedron Lett., 2010, 51, 2048–2051 CrossRef CAS PubMed; (c) R. Martinez, D. J. Ramon and M. Yus, Org. Biomol. Chem., 2009, 7, 2176–2181 RSC.
- (a) S. Wei and H. Du, J. Am. Chem. Soc., 2014, 136, 12261–12264 CrossRef CAS PubMed; (b) I. Chatterjee and M. Oestreich, Angew. Chem., Int. Ed., 2014 Search PubMed; (c) T. Mahdi and D. W. Stephan, J. Am. Chem. Soc., 2014, 136, 15809–15812 CrossRef CAS PubMed; (d) E. Blondiaux, J. Pouessel and T. Cantat, Angew. Chem., Int. Ed., 2014, 53, 12186–12190 CrossRef CAS PubMed; (e) L. Greb, C. G. Daniliuc, K. Bergander and J. Paradies, Angew. Chem., Int. Ed., 2013, 52, 5876–5879 CrossRef CAS PubMed; (f) S. J. Geier, P. A. Chase and D. W. Stephan, Chem. Commun., 2010, 46, 4884–4886 RSC; (g) P. A. Chase, T. Jurca and D. W. Stephan, Chem. Commun., 2008, 1701–1703 RSC.
- Q. Xu, Q. Li, X. Zhu and J. Chen, Adv. Synth. Catal., 2013, 355, 73–80 CrossRef CAS PubMed.
- Y. Gao, D. Ma, C. Wang, J. Guan and X. Bao, Chem. Commun., 2011, 47, 2432–2434 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: The details about the characterization of isolated compounds. See DOI: 10.1039/c5ra07681a |
| This journal is © The Royal Society of Chemistry 2015 |



