Ammonium iodide-promoted cyclization of ketones with DMSO and ammonium acetate for synthesis of substituted pyridines†
Abstract
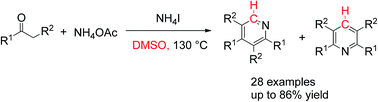
A simple and efficient method has been developed for the synthesis of symmetrical and unsymmetrical pyridines via NH4I-promoted cyclization of ketones with DMSO and NH4OAc. It was found that methyl ketones always gave selective formation of the unsymmetrical pyridine, while non-methyl ketones gave unpredictable results (symmetrical or non-symmetrical product only, or a mixture of the two). In addition, the deuterium-labeling experiments indicated that the C4 or C6 of the target product pyridine rings resulted from DMSO.

 Please wait while we load your content...
Please wait while we load your content...