Photocatalytic-degradation and reduction of organic compounds using SnO2 quantum dots (via a green route) under direct sunlight
Abstract
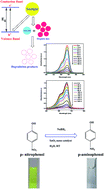
SnO2 Quantum Dots (QDs) were synthesized by a facile and green biological method using sugar cane juice. The biomolecules present in the juice act as complexing as well as capping agents. No other external agents are required for the production of the SnO2 Quantum Dots (QDs). This method resulted in the formation of spherical SnO2 QDs having a particle size of ∼3–4.5 nm with a tetragonal crystal structure. The biosynthesized SnO2 QDs were characterized by transmission electron microscopy (TEM), selected area electron diffraction (SAED), Energy Dispersive Spectroscopy (EDS) and Fourier transform infrared spectroscopy (FT-IR). The optical properties were investigated using UV-visible spectroscopy. In the electronic spectra, a clear blue shift in the band gap energy is observed with a decrease in particle size. This is because of three dimensional quantum confinement effects. The biosynthesized SnO2 QDs act as an efficient photocatalyst for the degradation of rose bengal and methylene blue dye under direct sunlight. The catalytic activity of the biosynthesized SnO2 QDs were also evaluated by the conversion of p-nitrophenol to p-aminophenol in the presence of NaBH4. The reaction was carried out in a green solvent i.e., water at room temperature. It was observed that 99.5% of p-nitrophenol was reduced in the presence of SnO2 QDs within 60 min. These investigations indicate that the biosynthesized SnO2 QDs may be effective and potential materials for the elimination of hazardous pollutants from wastewater.

 Please wait while we load your content...
Please wait while we load your content...