Magnetic ground state of nanosized β-Fe2O3 and its remarkable electronic features†
Abstract
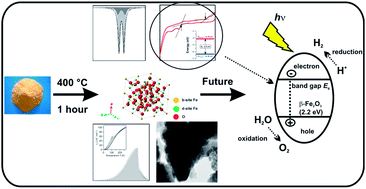
To date, iron oxides have been extensively investigated for promising high applicability in various scientific and industrial fields. In general, several forms can be distinguished with respect to their crystal structure, which drives their specific physical (in particular, magnetic) properties. In this study, the pure β-Fe2O3 phase, prepared in a nanoparticle form by a solid-state synthetic strategy, was investigated by employing 57Fe Mössbauer spectroscopy, magnetization measurements, transmission electron microscopy, X-ray powder diffraction, heat capacity measurements, and cyclic voltammetry. It is revealed that below the Néel transition temperature, β-Fe2O3 behaves as a canted antiferromagnet with a small net magnetic moment. For further possible utilization in photoelectrochemical applications, an estimation of the β-Fe2O3 band gap by cyclic voltammetry was performed, which was measured to be ∼2.2 eV.

 Please wait while we load your content...
Please wait while we load your content...