A rheological study on non-rubber component networks in natural rubber
Abstract
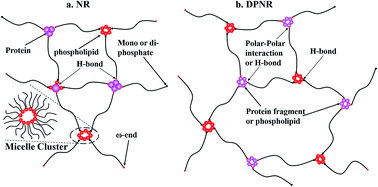
Phospholipids and proteins were separately removed as the main non-rubber components to individually study their effect on the structure and properties of the rubber. Fourier Transform Infrared Spectroscopy (FTIR) and 1H nuclear magnetic resonance spectroscopy (1H-NMR) were used to characterize the chemical structure and the residual non-rubber component. A rheology study and stress relaxation measurements were used to study the role that non-rubber components play in natural networks. A rheological study showed that natural rubber (NR) and deproteinized natural rubber (DPNR) exhibited similar dynamic modulus at 170 °C. The lack of superposition in van Gurp–Palmen (vGP) curves at different temperatures for NR and DPNR, together with the shape of vGP curves, proved that long chain branching was mainly constructed by phospholipids. Stress relaxation measurements at room temperature were fitted with the Maxwell model and showed that the NR relaxation curve underwent a quick decrease and then came to an equilibrium stress retention of 58%, which is about 3 times higher than that of DPNR, indicating that proteins in NR contributed to the network structure at room temperature. Combining molecular dynamic studies, the interaction of proteins and phospholipids in non-rubber networks was proposed.

 Please wait while we load your content...
Please wait while we load your content...