Regioselective synthesis of polygamma (γ) acid
Abstract
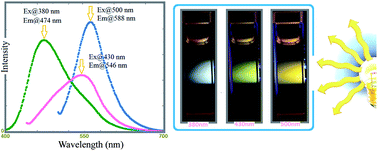
The oxidative polymerization of 6-amino-4-hydroxy-2-naphthalenesulfonic acid in an aqueous alkaline medium leads to the formation of a polygamma (γ) acid consisting of regioselective 1–4 oxazine rings. Spectral characterizations were performed by NMR, FT-IR, and UV-Vis techniques. The average molecular weight and polydispersity index of the gamma acid polymer (γ-AP) were found to be 32 000 Da and 1.42, respectively. γ-AP also presented an uncommon multicolored emission behavior in dimethylsulfoxide (DMSO). When excited at 405, 480, and 532 nm, γ-AP emitted blue, green and yellow light, respectively. X-ray diffraction (XRD), differential scanning calorimetry (DSC), dynamic mechanical analyses (DMA), and transmission electron microscopy (TEM) illustrated that γ-AP is composed of semicrystalline nanofiber structures with different lengths between 10–500 nm. The kinetic parameters related to the solid state thermal decomposition of γ-AP were determined using differential isoconversional methods (Friedman and Kissenger) and integral isoconversional methods (FWO, KAS, Tang, Starink and Bosewell). By using the Tang, KAS, FWO, Starink, Bosewell, Kissenger and Friedman methods, the average values of the activation energies were calculated to be 184.4, 184.4, 185.4, 188.5, 183.6, 179.7 and 187.1 kJ mol−1, respectively. The master plot curves also suggested a diffusion-type kinetic model for the solid state thermal decomposition stage of γ-AP.

 Please wait while we load your content...
Please wait while we load your content...