Multi-layered graphene quantum dots derived photodegradation mechanism of methylene blue†
Abstract
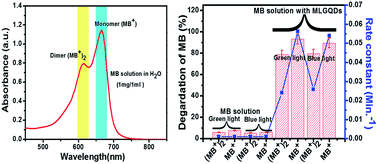
The photocatalytic degradation of methylene blue (MB) under visible light in the presence of a multi-layered graphene quantum dots (MLGQDs) photocatalyst has been investigated in aqueous heterogeneous solution. The photodegradation mechanism, following pseudo first order kinetics, was examined for the effects of the existing monomer (MB+)–dimer {(MB+)2} equilibrium in MB solution. The photocatalytic degradation efficiency of MB+ achieved 93.3% with a rate constant (k) of 0.056 min−1 after 60 min irradiation with green light, while less degradation ∼ 89.44% with k ∼ 0.024 min−1, was achieved for (MB+)2. MB+ is perhaps a short-lived species and favors the photodegradation of MB in comparison to the (MB+)2 species. Similar trends have been found under blue light irradiation. MB+ species easily pulls a proton from the functional groups of MLGQDs, resulting in an intermediate product Luco-methylene blue (LMB). Finally, all MB species and intermediate products degrade into an environmental benign product via highly reactive OH radicals. In addition, our ab initio theoretical results reveals that monomers abstract a proton from hydroxyl groups of MLGQDs and formation of LMB takes place, which is weakly bonded with MLGQDs by hydrogen bonds.

 Please wait while we load your content...
Please wait while we load your content...