2D hybrid anode based on SnS nanosheet bonded with graphene to enhance electrochemical performance for lithium-ion batteries†
Shuankui Liab,
Jiaxin Zhengb,
Shiyong Zuoa,
Zhiguo Wu*a,
Pengxun Yana and
Feng Pan*b
aSchool of Physical Science and Technology, Lanzhou University, Lanzhou, Gansu 730000, China. E-mail: zgwu@lzu.edu.cn; Tel: +86-931-8912719
bSchool of Advanced Materials, Peking University Shenzhen Graduate School, Shenzhen 518055, China. E-mail: panfeng@pkusz.edu.cn; Tel: +86-755-26033200
First published on 7th May 2015
Abstract
A hybrid anode based on SnS nanosheets bonded with reduced graphene oxide (SnS NS/RGO) and synthesized with graphene oxide is formed through a facile solvothermal method. It is found that the 2D SnS nanosheets with few layers are well dispersed on the crumpled reduced graphene oxide surface. Electrochemical tests indicate that the SnS NS/RGO hybrid exhibits a very high reversible capacity (791 mA h g−1) with excellent cycle stability and significantly enhanced rate capability. The factors contributing to the enhanced electrochemical performance of the hybrid anode can be ascribed to the chemically bonded interface between SnS NS and RGO, creating effective charge transportation with electron coupling in the novel two-dimensional composite nanostructure. This work provides insights into the structural design of SnS-based hybrid electrode materials, which will be important for the future development of high performance electrode materials.
Introduction
Li-ion batteries (LIBs) are the most used renewable energy source for all kinds of portable electronic devices owing to their excellent energy conversion efficiency and high energy density.1–5 However, the anode material in commercially available Li-ion batteries is typically graphite, which exhibits a low theoretical lithium storage capacity of around 370 mA h g−1 and poor rate capability.6–8 To meet the continuous demands for electrochemical energy storage, high performance electrode materials for the next generation of LIBs, especially anode materials, such as Si,9 tin-based compounds,10 and metal oxides11 have been under intense research during the past decade. Tin-based compounds are one of the promising anode materials for lithium ion batteries due to their low cost, high theoretical capacity, and widespread availability. Stannous sulfide (SnS) is an important tin-based compound with a high theoretical capacity (782 mA h g−1 based on 4.4 Li per molecule), which is considered as a particularly interesting anode material for LIBs. However, the practical application of the SnS anode is still a challenge due to its poor electron transport and slow Li-ion diffusion in the electrodes, which leads to poor cycling and rate performance.12–14 Various SnS nanostructures, such as 1D nanowires, 2D nanosheets,15–17 and 3D SnS nanoflowers,13 have been extensively studied in order to improve the electrochemical performance by shortening Li-ion and electron diffusion paths, enlarging the electrode/electrolyte interfacial area as well as facilitating strain relaxation during the insertion/extraction processes. However, the electrochemical performance of these SnS nanostructures is still limited by their extremely low electrical conductivity. The design and fabrication of SnS based electrodes with high specific capacity and high-rate capability is still a great challenge.18,19The combination of SnS with conducting agents to form SnS-based hybrids, has been regarded as one of effective approaches to circumvent the above issues.20,21 Graphene, a new 2D carbon material, is considered an ideal matrix to support 2D SnS nanosheets, not only due to its appealing characteristics such as a large specific surface area, superior electrical conductivity, and excellent structural flexibility, but also due to its 2D layered structure which is beneficial for forming well aligned layered composites.22 In our previous work, we found that the depolarization effect of the hybrid electrode, with the cathode (such as Li2FeSiO4 (LFS)) chemically bonded to graphene, was due to the electron coupling at the interface between cathode (nano-LFS) and graphene creating effective charge transportation so improving the electrochemical performance.23 Consequently, modification of SnS anode material with graphene has attracted broad interest, which is expected to largely enhance its electrochemical performance. The synergy between the functions of the two materials, the high capacity of SnS and the good electrical conductivity of graphene, to generate high lithium ion diffusion efficiency can be exploited in the SnS nanosheet/graphene hybrids to yield high performance anodes in Li-ion batteries.12 However, very few reports study the electrochemical performance of uniform 2D SnS nanosheet chemically bonded with graphene as a hybrid anode material for lithium ion batteries.
Herein, we report a facile solvothermal method to grow SnS nanosheets (NS) on reduced graphene oxide (RGO) nanosheets to form SnS NS/RGO hybrids. The as-prepared hybrid exhibits a very high reversible capacity (as high as 791 mA h g−1) with excellent cycle stability and good rate capability. The well aligned layered structures for the SnS NS/RGO hybrids and the well coupled interface between SnS NS and graphene ensure the synergy between the functions of the two materials, the high capacity of SnS and the good electrical conductivity of graphene, thus yielding high electrochemical performance batteries.
Experimental section
Preparation of SnS NS/RGO hybrid: natural graphite powder was oxidized to graphite oxide (GO) by the modified Hummers’ method. The SnS NS/RGO hybrid was prepared by a solvothermal route. In a typical synthesis of the SnS NSs/RGO hybrid, 20 mg of graphite oxide was dispersed into 30 ml ethylene glycol solution by ultra-sonication for 60 min. Then, 226 mg SnCl2·2H2O (1 mmol) and 210 mg citric acid were added to the solution and then stirred for 60 min. 2 ml (NH3)2S was added to the solution and the final solution was further stirred for 5 min, and then transferred to a 40 ml Teflon-lined stainless steel autoclave. After heating at 190 °C for 18 h and naturally cooled to room temperature, the black product was filtered and washed several times with deionized water and absolute ethanol, and then dried at 60 °C for 24 h to obtain the two-dimensional (2D) hybrid SnS nanosheet/reduced graphene oxide. Pure SnS was fabricated without the graphite oxide as a comparison.Electrochemical measurements
R2016 type half cells were assembled with the prepared hybrids as the anode material in a high-purity argon-filled glove box. The anodes were prepared by mixing the active materials, carbon black, and polyvinylidene difluoride (PVDF) at a weight ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 in NMP (N-methyl-2-pyrrolidone) solvent to form a slurry. The typical loading density on a Cu foil was ∼1.5 mg cm−2. A Li foil was used as the cathode. LiPF6 (1 M) in ethylene carbonate (EC)–diethyl carbonate (DEC) (1
10 in NMP (N-methyl-2-pyrrolidone) solvent to form a slurry. The typical loading density on a Cu foil was ∼1.5 mg cm−2. A Li foil was used as the cathode. LiPF6 (1 M) in ethylene carbonate (EC)–diethyl carbonate (DEC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) was used as the electrolyte. The charge–discharge tests were performed on a multi-channel battery workstation (LAND-CT2001C). Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) was performed on a CHI660E electrochemical workstation.
1 w/w) was used as the electrolyte. The charge–discharge tests were performed on a multi-channel battery workstation (LAND-CT2001C). Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) was performed on a CHI660E electrochemical workstation.
Characterization
The morphology and structure were observed by scanning electron microscopy (SEM, Hitachi S-4800) with an acceleration voltage of 5 kV, and transmission electron microscopy (TEM: JEM-2100) operated at 200 kV. The crystal structures of the as-synthesized products were characterized by X-ray diffraction (XRD) on a Philips X’Pert Pro Diffractometer, using Cuα1 radiation (λ = 1.54056 Å). The X-ray photoelectron spectroscopy (XPS) data were determined on Thermo Scientific XPS spectrometers.Results and discussion
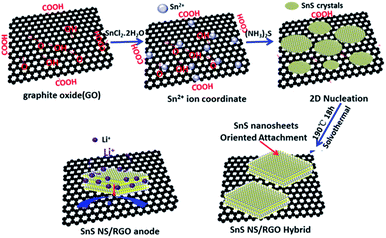
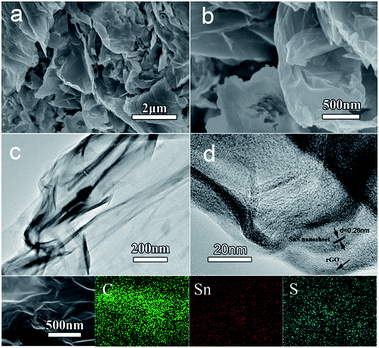
The fabrication process of the SnS NS/RGO is illustrated in Scheme 1. At the early stage, Sn2+ ions can be very easily absorbed on GO by the coordinate interaction between the oxygen-containing functional groups (–OH and –COOH) on GO and the Sn2+ ions to form chemical bonds. Therefore, as the (NH3)2S was added to the solution, the nucleation and subsequent growth of SnS is selective on the GO surfaces with little free particle growth in solution. Subsequently, under the solvothermal conditions, SnS crystals are grown according to its growth habit to further reduce the interface energy between primary nanoparticles. In the case of the orthorhombic structure of SnS, the (100) facets are the most stable facets due to the strongest ionic interactions. Accordingly, it is rational that the SnS nanostructure tends to expose (100) facets. The 2-dimensional nucleation and growth of SnS on the GO surfaces leads to the formation of the SnS NS/RGO hybrid. It should be pointed out that the initial GO will be reduced under the solvothermal conditions.24 The structured SnS NS/RGO hybrid electrode is designed with the following advantages: (1) ultrathin plate-like SnS subunits to facilitate fast Li+ diffusion and alleviate pulverization, (2) highly conductive RGO framework to enhance electrical conductivity of the electrode, (3) well aligned layered structures and a well coupled interface between SnS NS and graphene which are beneficial to effective and rapid charge transfer from the ultrathin SnS layers to the RGO, (4) large surface area of RGO framework to improve electrolyte wettability and relieve the mechanical stress and accommodate large volume changes.25The morphology and microstructure of the as-prepared SnS NS/RGO hybrid were studied by field emission scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The SEM image in Fig. 1a reveals the 2D morphology of the SnS NS/RGO(D10) hybrid. Abundant irregular SnS nanosheets with a thickness of about 10–20 nm are clearly observed in the SEM image. It is should be noted that the SnS nanosheets (Fig. 1b) are more rigid and thicker than the RGO nanosheets. The TEM image in Fig. 1c further clearly shows curved SnS nanosheets dispersed on large RGO substrates, indicating the formation of a hybrid SnS nanosheet-RGO heterostructure. Furthermore, the as-prepared SnS NS/RGO(D10) architecture possesses a hybrid structure with interconnected pores, which can effectively accommodate large volume changes, thus leading to improved lithium-storage properties. As shown in Fig. 1d, the SnS shows a well-layered structure with interactions, with an interlayer distance of the (400) plane of 0.28 nm, supported on the RGO surface. It is well known that the orthorhombic SnS phase has a layered structure along the b-axis. The bonding within the layer is dominated by covalent bonding between Sn and S atoms, while the bonding between the layers, separated by 2.668 Å, is maintained by the weak van der Waals force. The thickness of the nanosheets is about 5–10 nm; this indicates that each sheet is composed of 20–40 layers of SnS along the b-axis. Owing to this weakly bound interlayer characteristic, the attachment of atoms on the lattice along the b-axis, during synthesis, generates such a high free energy that the crystal growth along this direction proceeds slowly, leading to the formation of anisotropic 2D nanosheets.24 The partial overlapping or coalescing of flexible nanosheets is likely to have originated from the cross-linking of the functional groups in the GO sheets, and thus, the SnS nanosheets are much easier to efficiently combine with GO.
 | ||
| Fig. 1 SEM and HRTEM images of the as-synthesized SnS NS/RGO(D10). (a) and (b) SEM images. (c) and (d) TEM images. | ||
Fig. 2a shows the XRD patterns of the hybrid SnS NSs/RGO (D10) and bare SnS. The diffractogram of SnS also matched well with the reported JCPDS data of orthorhombic SnS (space group: Pbnm, JCPDS 39-0354) with lattice constants of a = 4.3291 Å, b = 11.1923 Å, and c = 3.9838 Å. Because of the low amount and the relatively low diffraction intensity of RGO in the composites, no characteristic diffraction of carbon species can be observed after the introduction of GO. Fig. 2b compares the Raman spectra of SnS NS/RGO(D10) and GO; two typical carbon peaks are observed. The peak at about 1589 cm−1 (G band) is related to the vibration of the sp2-bonded carbon atoms in a 2-dimensional hexagonal lattice, while the peak at about 1326 cm−1 (D band) is related to the defects and disorder in the hexagonal graphitic layers. The intensity ratio of the D band to G band (ID/IG) of SnS NS/RGO is calculated as 1.16, which is higher than the intensity ratio in GO. The slight enhancement of the intensity ratio in the SnS NS/RGO(D10) can be attributed to the increase of the disorder in the GO resulting from the aggressive solvothermal reaction and the loading of SnS nanosheets.
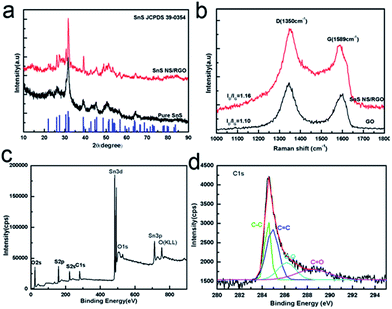 | ||
| Fig. 2 (a) XRD curves and (b) Raman spectrum of the SnS NS/RGO composite. XPS spectra of SnS NS/RGO hybrid: (c) survey; (d) C 1s. | ||
To further evaluate the SnS NS/RGO(D10) composite, X-ray photoelectron spectroscopy (XPS) was performed, as shown in Fig. 2c. The two strong peaks at around 486.8 and 495.4 eV (Fig. S1c†) can be attributed to Sn 3d5/2 and 3d3/2 respectively, which agrees well with the reference data of Sn2+ in SnS. No evidence of Sn4+ (binding energy at 485.9 eV) was detected in the spectra. The S2p3/2 at 161.83 eV (Fig. S1d†) and S2s1/2 at 223.51 eV are attributed to the binding energies of SnS, which demonstrates that there is no elemental sulfur (164.05 eV in binding energy) present in the sample.26 The C 1s peak (284.5 eV) corresponding to the C–C bonding (sp2 carbon) in graphite is very strong. The peak arising from the oxygenated carbons (carbon in C–O at 286.2 eV; carbonyl carbon, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, 287.9 eV) was also observed, suggesting that the oxygen-containing (C–O, C
O, 287.9 eV) was also observed, suggesting that the oxygen-containing (C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) functional groups on the surfaces of the GO chemically bonded with Sn of SnS to generate interfacial bonding (–C–O–Sn–) as shown in Fig. 2d. Under the solvothermal condition, the increased temperature and autogenous pressure led to the reduction of graphene oxide into RGO, but the C–O or C
O) functional groups on the surfaces of the GO chemically bonded with Sn of SnS to generate interfacial bonding (–C–O–Sn–) as shown in Fig. 2d. Under the solvothermal condition, the increased temperature and autogenous pressure led to the reduction of graphene oxide into RGO, but the C–O or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of the interfacial bonding (–C–O–Sn–) was still left after the reduction.
O of the interfacial bonding (–C–O–Sn–) was still left after the reduction.
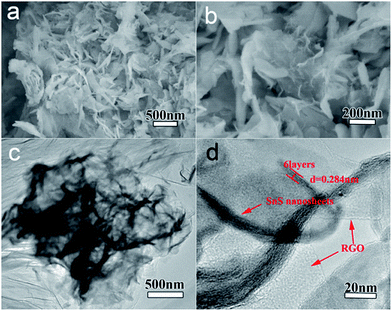
To compare with SnS NS/RGO, the pure SnS prepared without the graphite oxide had a similar morphology. As shown in Fig. S2,† many irregular nanosheets with a thickness of about 10 nm were clearly observed. Another kind of SnS NS/RGO composite (SnS NS/RGO(L5)) fabricated under a low concentration of the Sn2+ source (the amount of SnCl2·2H2O was reduced to 0.5 mmol), is shown in Fig. 3. The composite also has a 2-dimensional nanoflake structure (Fig. 3a–c). The high-resolution TEM (HRTEM) image shows that SnS layers with an interlayer distance of 0.28 nm were grown on the surface of the GO (Fig. 3d) and the thickness of SnS (about 4–5 layers) grown on the surface of the GO was quite thinner than that of SnS NS/RGO(D10). To verify the 2-dimensional nanoflake structure of SnS layers grown on the surface of RGO, EDX elemental mappings (Fig. 3) of the nanosheet were obtained. The result clearly indicates that there is a uniform dispersion of the element C, Sn, S in the nanosheet. The carbon content of SnS NS/RGO (D10) and (L5) determined by thermogravimetric analysis (TGA) (Fig. S3†) was 17.9 and 44.6 wt%, respectively, indicating the former sample had a higher loading ratio of SnS nanosheets on the RGO surfaces.
It is well known that the lithium intercalation and conversion reactions of a Sn-based anode material are based on the formation of metallic Sn and subsequent generation of a Li–Sn alloy:27
| SnS + xLi+ + xe− → Li2S + Sn | (1) |
| Sn + xLi+ + xe− ↔ LixSn (0 ≤ x ≤ 4.4) | (2) |
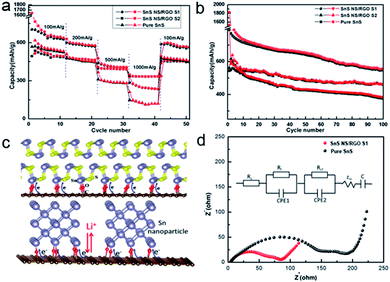
Cyclic voltammetric (CV) experiments were carried out to understand the electrochemical reactive process of the SnS/RGO hybrids in the range of 0.02–1.5 V for five cycles at a scan rate of 0.2 mV s−1. The broad peak located at around 1 V in the first cathodic scan corresponds to the reduction of SnS to metallic Sn accompanying the formation of Li2S and the solid electrolyte interphase (SEI) film, as shown in eqn (1), and similar results have previously been reported for other Sn-based anode materials.26,27 The decomposition of the SnS into metallic Sn and Li2S as well as the formation of a solid electrolyte interface (SEI) may lead to the large irreversibility of SnS-based anodes in the first charge–discharge cycle, which is generally consistent with those reported in literature. The more cathodic potential at around 0.23 V and the anodic potential at about 0.5 V in the first scan can be attributed to the alloying (cathodic scan) and dealloying (anodic scan) processes. From the second cycle onwards, the CV curves mostly overlap, indicating the good reversibility of the electrochemical reactions. In agreement with this CV result, two poorly defined plateaus can be identified in the discharge voltage profiles (Fig. 4b).27
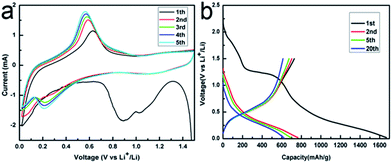 | ||
| Fig. 4 (a) Cycle voltammogram of the SnS NS/RGO S1 electrode at a scan rate of 0.2 mV s−1. (b) The 1st, 2nd, 5th and 20th discharge–charge profiles at a current density of 100 mA g−1. | ||
Benefitting from the unique 2D hybrid structure and the presence of RGO, SnS NS/RGO exhibits a higher rate performance than the pure SnS nanosheet. For SnS NS/RGO(D10), with about 10 nm thickness of SnS, when the current densities increase from 100 to 200, 500 and 1000 mA g−1, the electrode exhibits high capacity retention, as the specific capacities change from 785 to 627, 477, and 331 mA h g−1, respectively. Remarkably, when the current rate is reduced back to 100 mA g−1 after 50 cycles, a stable high discharge capacity of about 595 mA h g−1 can be recovered, indicating the high rate capability and good cycling stability of SnS NS/RGO as an anode material. In addition, it is noteworthy that at a higher current density of 1000 mA g−1, SnS NS/RGO(L5), with about 4–5 layers SnS, was still able to deliver a discharge capacity of 360 mA h g−1, which is much higher than that of SnS NS/RGO(D10) with about 20–30 layers SnS. This might arise from the difference in the RGO content of the 2D hybrids and the thinner layer of SnS. The large content of RGO is not only beneficial for the electrical transportation, but also can accommodate large volume changes. Furthermore, electrochemical impedance spectroscopy (EIS) measurements were carried out for SnS NS/RGO (D10) and pure SnS electrodes, after several cycles, as shown in the Nyquist plot (Fig. 5d). The values of Rf and Rct for the SnS NS/RGO(D10) hybrid (65 Ω, 83 Ω) are much lower than pure SnS (69 Ω, 174 Ω), indicating that charge carriers could be effectively and rapidly conducted back and forth from ultrathin SnS layers to the RGO layers. This can be attributed to good electron coupling between SnS NS and RGO (Fig. 5c) which makes SnS NS feel direct electric-potential without the I–R (current–resistance) deduction, creating the depolarization effect and enhancing the electrochemical activity of SnS NS. As a result, charge carriers could be effectively and rapidly transported back and forth from the SnS layers to RGO layers.
Fig. 5b shows the discharge–charge cycling performance of the above mentioned anode materials, evaluated between 0.05 and 1.5 V at a current density of 100 mA g−1. The SnS NS/RGO (D10) and (L5) exhibit very high initial capacities of 1625 and 1535 mA h g−1, respectively. These high initial capacities may be partly attributed to both the presence of defects in the graphene and the disorder of the unique 2D structure of SnS. The initial discharge capacities of SnS NS/RGO (D10) and SnS NS/RGO (L5) were 791 and 601 mA h g−1, which is close to the theoretical specific capacity of SnS (790 mA h g−1). The irreversible capacity loss may be mainly attributed to irreversible reaction in eqn (1) and the inevitable formation of a solid electrolyte interface (SEI) film, which are common to most Sn-based anode materials. After 100 cycles, the capacities of SnS NS/RGO (D10) and (L5) are stable at about 560 and 460 mA h g−1, delivering 73.7% and 76.5% of the second cycle capacity (791 and 601 mA h g−1) (Fig. 5b). Whereas for the pure SnS nanosheet, the capacity decays much faster to less than 400 mA h g−1 within 100 cycles, which could be ascribed to the morphology being inevitably damaged and resulting in aggregation and pulverization after a long time cycling. It should be noted that compared with SnS NS/RGO (D10), (L5) has a lower capacity but better cyclic performance, which could be mainly attributed to the difference in the RGO content (17.9 and 44.6 wt% for S1 and S2). Remarkably, the sample of SnS NS/RGO (D10) even demonstrates excellent cyclic capacity retention at a higher current rate (Fig. S4†). At the end of 100 charge–discharge cycles, a reversible capacity of 520 and 390 mA h g−1 can be retained at current density of 200 and 500 mA g−1, indicating the high rate capability and good cycling stability of SnS NS/RGO as an anode material.
Compared with the previous results for SnS based materials as anodes for LIBs, the excellent electrochemical performance of the 2D hybrid SnS nanosheet/reduced graphene oxide electrode provides great potential as high-performance electrode materials for lithium-ion batteries, as shown in Table S1.† The excellent cycling stability and rate capability of the SnS NS/RGO hybrids could be attributed to the synergy between the functions of the two materials and the novel two-dimensional composite nanostructure. First, the highly conductive graphene oxide supplies 2D electronically conducting networks for the SnS NS/RGO composites to facilitate charge transfer. Second, the depolarization effect induced by a high level of electron coupling between SnS NS and the reduced graphene oxide makes the SnS NS fully electrochemically active.28 Third, the large surface area of the ultrathin SnS subunits with a preferential a-axis orientation leads to more reactive sites and a large contact area between the electrode material and electrolyte, which finally endows the composite with a high specific capacity.29 Finally, the highly conductive and flexible RGO can effectively accommodate large volume changes.
Conclusion
In summary, we have successfully fabricated SnS NS/RGO hybrids by directly growing ultrathin SnS nanosheets onto GO then reducing to RGO via a facile solvothermal process. The SnS NS/RGO hybrids exhibit higher lithium storage capacities and better cycling performance compared to bare SnS due to the rational design and engineering of the unique nanostructure and composition. The unique ultrathin sheet-on-sheet nanostructure not only offers 2D conducting networks but also facilitates rapid Li-ion transport, leading to enhanced rate capability. The present results suggest that SnS NS/RGO hybrids can be used as a promising anode material for high performance Li-ion batteries.Acknowledgements
The research was financially supported by Guangdong Innovation Team Project (no. 2013N080), Shenzhen Science and Technology Research Grant (no. ZDSY20130331145131323, CXZZ20120829172325895, JCYJ20120614150338154), and the National Natural Science Foundation of China (no. 11204114).Notes and references
- A. S. Arico, P. Bruce, B. Scrosati, J.-M. Tarascon and W. van Schalkwijk, Nat. Mater., 2005, 4, 366 CrossRef CAS PubMed.
- N. Kamaya, K. Homma, Y. Yamakawa, M. Hirayama, R. Kanno, M. Yonemura, T. Kamiyama, Y. Kato, S. Hama, K. Kawamoto and A. Mitsui, Nat. Mater., 2011, 10, 682–686 CrossRef CAS PubMed.
- Y. Idota, T. Kubota, A. Matsufuji, Y. Maekawa and T. Miyasaka, Science, 1997, 276, 1395–1399 CrossRef CAS.
- M. G. Kim and J. Cho, Adv. Funct. Mater., 2009, 19, 1497–1514 CrossRef CAS PubMed.
- M. Armand and J.-M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS PubMed.
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359–367 CrossRef CAS PubMed.
- Z. S. Wu, W. C. Ren, L. Wen, L. B. Gao, J. P. Zhao, Z. P. Chen, G. M. Zhou, F. Li and H. M. Cheng, ACS Nano, 2010, 4, 3187–3194 CrossRef CAS PubMed.
- X. Y. Han, G. Y. Qing, J. T. Sun and T. L. Sun, Angew. Chem., Int. Ed., 2012, 51, 1–6 CrossRef PubMed.
- K. Fu, O. Yildiz, H. Bhanushali, Y. X. Wang, K. Stano, L. G. Xue, X. W. Zhang and P. D. Bradford, Adv. Mater., 2013, 25, 5109–5114 CrossRef CAS PubMed.
- C.-M. Wang, W. Xu, J. Liu, J.-G. Zhang, L. V. Saraf, B. W. Arey, D. Choi, Z.-G. Yang, J. Xiao, S. Thevuthasan and D. R. Baer, Nano Lett., 2011, 11, 1874–1880 CrossRef CAS PubMed.
- L. Chen, H. Y. Xu, L. Li, F. F. Wu, J. Yang and Y. T. Qian, J. Power Sources, 2014, 245, 429–435 CrossRef CAS PubMed.
- S. K. Li, S. Y. Zuo, Z. G. Wu, Y. Liu, R. F. Zhuo, J. J. Feng, D. Yan, J. Wang and P. X. Yan, Electrochim. Acta, 2014, 1, 355–362 CrossRef PubMed.
- D. D. Vaughn II, O. D. Hentz, S. Chen, D. Wang and R. E. Schaak, Chem. Commun., 2012, 48, 5608–5610 RSC.
- Y. J. Zhang, J. Lu, S. L. Shen, H. R. Xu and Q. B. Wang, Chem. Commun., 2011, 47, 5226–5228 RSC.
- J. Lu, C. Y. Nan, L. H. Li, Q. Peng and Y. D. Li, Nano Res., 2013, 6(1), 55–64 CrossRef CAS PubMed.
- G. G. Kumar, K. Reddy, K. S. Nahma, N. Angulakshmi and A. M. Stephan, J. Phys. Chem. Solids, 2012, 73, 1187–1190 CrossRef PubMed.
- J.-G. Kang, J.-G. Park and D.-W. Kim, Electrochem. Commun., 2010, 12, 307–310 CrossRef CAS PubMed.
- H. C. Tao, X. L. Yang, L. L. Zhang and S. B. Ni, J. Electroanal. Chem., 2014, 728, 134–139 CrossRef CAS PubMed.
- J. S. Zhu, D. L. Wang and T. F. Liu, Ionics, 2014, 20, 141–144 CrossRef CAS PubMed.
- D. B. Kong, H. Y. He, Q. Song, B. Wang, Q. H. Yang and L. J. Zhi, RSC Adv., 2014, 4, 23372–23376 RSC.
- C. Zhai, N. Du and H. Z. D. Yang, Chem. Commun., 2011, 47, 1270–1272 RSC.
- F. Bonaccorso, L. Colombo, G. Yu, M. Stoller, V. Tozzini, A. C. Ferrari and V. Pellegrini, Science, 2015, 347(6217), 1246501 CrossRef PubMed.
- J. L. Yang, L. Hu, J. X. Zheng, D. P. He, L. L. Tian, S. C. Mu and F. Pan, J. Mater. Chem. A, 2015, 3, 9601–9608 CAS.
- E. M. Lotfabad, J. Ding, K. Cui, A. Kohandehghan, W. P. Kalisvaart, M. Hazelton and D. Mitlin, ACS Nano, 2014, 8, 7115–7129 CrossRef CAS PubMed.
- J. Wang, J. L. Liu, D. L. Chao, J. X. Yan, J. Y. Lin and Z. X. Shen, Adv. Mater., 2013, 26(42), 7162–7169 CrossRef PubMed.
- P. Jain and P. Arun, J. Appl. Phys., 2014, 115, 204512 CrossRef PubMed.
- H. B. Wu, J. S. Chen, H. H. Hng and X. W. Lou, Nanoscale, 2012, 4, 2526–2542 RSC.
- G. M. Zhou, L. Li, Q. Zhang, N. Li and F. Li, Phys. Chem. Chem. Phys., 2013, 15, 5582–5587 RSC.
- X. Huang, C. L. Tan, Z. Y. Yin and H. Zhang, Adv. Mater., 2014, 26(14), 2185–2204 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra07292a |
| This journal is © The Royal Society of Chemistry 2015 |