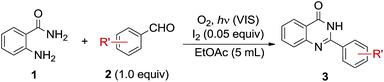Aerobic photooxidative synthesis of 2-aryl-4-quinazolinones from aromatic aldehydes and aminobenzamide using catalytic amounts of molecular iodine†
Y. Nagasawaa,
Y. Matsusakia,
T. Nobutaa,
N. Tadaa,
T. Miurab and
A. Itoh*a
aGifu Pharmaceutical University, 1-25-4, Daigaku-nishi, Gifu 501-1196, Japan. E-mail: itoha@gifu-pu.ac.jp
bTokyo University of Pharmacy and Life Sciences, 1432-1 Horinouchi, Hachioji, Tokyo 192-0392, Japan
First published on 21st July 2015
Abstract
This study reports a safe, mild, and environmentally benign synthetic method toward 2-aryl-4-quinazolinones from aromatic aldehydes and aminobenzamides through a cyclization–oxidation sequence using a catalytic amount of iodine, which serves as both a Lewis acid and an oxidant, harmless visible light irradiation, and molecular oxygen as the terminal oxidant.
4-Quinazolinones are well-known compounds with a variety of biological and pharmacological activities, such as anticancer, anti-inflammatory, and antidiuretic, and are assigned as privileged structures in drug discovery.1 As such, many methods have been reported for the synthesis of 4-quinazolinones.2 Among those reported, oxidation of cyclic aminal intermediates obtained from 2-aminobenzamides and aldehydes with I2,3 DDQ (dichlorodicyanobenzoquinone),4 CuCl2,5 CuBr,6 MnO2,7 KMnO4,8 FeCl3·6H2O,9 or microwave irradiation conditions10,4b are reliable methods; however, they require stoichiometric amounts of reagents, transition metals, or involve a complicated procedure.
In recent times, iodine source catalyzed C–H oxidation with various stoichiometric oxidants, such as H2O2 or tert-BuOOH, and has attracted great interest because iodine sources have low toxicity and are inexpensive compared with transition metal catalysts.11 Furthermore, iodine serves as both a Lewis acid and an oxidant.12 Therefore, in multi-step syntheses, iodine is able to accelerate stoichiometric or catalytic domino reactions.13
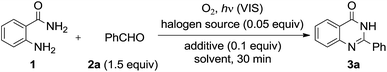
From the perspective of green chemistry, various aerobic photooxidative reactions using catalytic amounts of iodine sources with molecular oxygen as the terminal oxidant under visible light irradiation have been developed in our laboratory.14 The methods to synthesize the quinazolinones using molecular oxygen were recently reported but they require transition metals or thermal condition.15 As such, we developed an aerobic photooxidative synthesis of 2-aryl-4-quinazolinones from aromatic aldehydes and aminobenzamide using a catalytic amount of iodine with more mild condition (Scheme 1). Herein we describe this reaction in detail.
We selected 2-aminobenzamide (1) and benzaldehyde (2a) as the test substrates for optimization of the reaction conditions (Table 1). Although we examined the reaction conditions with various halogen sources, the yields of 2-phenyl-4-quinazolinone (3a) were unsatisfactory, except for those using molecular iodine (entries 1–6). In this reaction, addition of K2CO3 or Na2SO4 had only a marginal effect (entries 13 and 14), while addition of AcOH decreased the yield of 3a to 17% (entry 15). Further study of the solvent, amount of benzaldehyde, and reaction time revealed that using iodine (0.05 equiv.) and benzaldehyde (1.0 equiv.) in EtOAc for 1 h gave the best yield (entry 17). The fact that 3a was not obtained or was obtained only in low yield without iodine, molecular oxygen, or photoirradiation shows the necessity of these conditions for this reaction (entries 18–20). Under these conditions, 1 and the cyclized intermediate (2,3-dihydro-2-phenyl-4(1H)-quinazolinone: 4a) were obtained in low yields.
| Entry | Halogen source | Additive | Solvent | Yield (%)a |
|---|---|---|---|---|
| a The reaction conditions: 1 (0.3 mmol), 2a (1.5 equiv.), and halogen sources (0.05 equiv.) in solvent (5 mL) was stirred and irradiated with fluorescent lamp under O2 atmosphere for 30 min. 1H NMR yields.b Reaction was conducted for 1 h and conducted with 1.0 equiv. of benzaldehyde.c The number in the parentheses is the isolated yield.d 1 (26%) was recovered with 4a (16%).e Reaction was conducted under argon. 1 (36%) was recovered with 4a (18%).f Reaction was conducted in the dark. 1 (18%) was recovered with 4a (28%). | ||||
| 1 | KI | — | EtOAc | 0 |
| 2 | LiI | — | EtOAc | 0 |
| 3 | CaI2 | — | EtOAc | 0 |
| 4 | ZnI2 | — | EtOAc | 6 |
| 5 | I2 | — | EtOAc | 86 |
| 6 | I2 | — | MeOH | 74 |
| 7 | I2 | — | i-Pr2O | 24 |
| 8 | I2 | — | Hexane | 22 |
| 9 | I2 | — | H2O | 11 |
| 10 | I2 | — | MeCN | 6 |
| 11 | I2 | — | Acetone | 0 |
| 12 | I2 | K2CO3 | EtOAc | 76 |
| 13 | I2 | Na2SO4 | EtOAc | 73 |
| 14 | I2 | AcOH | EtOAc | 17 |
| 15b | I2 | — | EtOAc | 85 (86)c |
| 16d | — | — | EtOAc | 0 |
| 17e | I2 | — | EtOAc | 0 |
| 18f | I2 | — | EtOAc | Trace |
Table 2 shows the results of the scope and limitations of photooxidative synthesis of 2-aryl-4-quinazolinones from various aromatic aldehydes under the optimized conditions. In general, the corresponding 2-aryl-4-quinazolinones were obtained in moderate to high yields regardless of the electron donating or weak electron withdrawing groups on the benzene ring of the aldehydes (entries 1–8). In contrast, 4-cyano and 4-nitrobenzaldehyde bearing strong electron withdrawing groups gave moderate and low yields of 3. Unfortunately, aliphatic aldehydes such as cyclohexanecarboxaldehyde and dodecyl aldehyde were poor substrates.
| Entry | R′ | Time (h) | Product | Yield (%)a | |
|---|---|---|---|---|---|
| a The reaction conditions: 1 (0.3 mmol), 2 (1.0 equiv.), and I2 (0.05 equiv.) in EtOAc (5 mL) was stirred and irradiated with fluorescent lamp under O2 atmosphere. Isolated yields. | |||||
| 1 | H | 2a | 1 | 3a | 86 |
| 2 | 4-OH | 2b | 5 | 3b | 80 |
| 3 | 4-OMe | 2c | 1 | 3c | 75 |
| 4 | 3-OMe | 2d | 1 | 3d | 81 |
| 5 | 4-Me | 2e | 5 | 3e | 76 |
| 6 | 3-Me | 2f | 10 | 3f | 93 |
| 7 | 4-tBu | 2g | 3 | 3g | 83 |
| 8 | 4-F | 2h | 15 | 3h | 85 |
| 9 | 4-Cl | 2i | 15 | 3i | 88 |
| 10 | 4-Br | 2j | 15 | 3j | 82 |
| 11 | 4-CF3 | 2k | 15 | 3k | 76 |
| 12 | 4-CN | 2l | 5 | 3l | 66 |
| 13 | 4-NO2 | 2m | 5 | 3m | 69 |
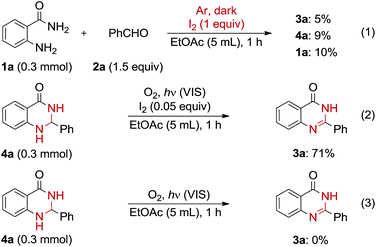
To clarify the reaction mechanism, several control experiments were examined. When one equivalent of molecular iodine was used in the absence of molecular oxygen and visible light irradiation, 3a was obtained in low yield and many by-products were formed (Scheme 2, eqn (1)). This result indicated that the reaction required irradiation with visible light and the presence of molecular oxygen. When 4a was used as a substrate under the optimal conditions, 3a was obtained in good yield (Scheme 2, eqn (2)). In contrast, no 3a was obtained in the absence of iodine (Scheme 2, eqn (3)). These results and entries 18–20 in Table 2 suggest that 4a is the reaction intermediate and molecular oxygen, iodine, and visible light are all required for the oxidation of 4a. Hypoiodous acid (IOH) may be the active species in the final step of the oxidation because it is easily prepared from an iodine source and a peroxide.16
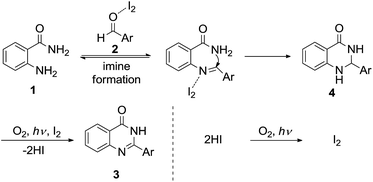
Scheme 3 shows a plausible path for this reaction. The intermediates (4) are formed through condensation between 2-aminobenzamides (1) and aldehydes (2), catalyzed by molecular iodine as a Lewis acid. Intermediate 4 is then transformed to 4-quinazolinones (3) by oxidation with molecular iodine, molecular oxygen, and visible light. In addition, hydrogen iodide, generated by the oxidation of 4, is reoxidized to molecular iodine under the aerobic photooxidative conditions.
Conclusions
In conclusion, we have developed an environmentally benign synthesis of 2-aryl-4-quinazolinones with a catalytic amount of iodine under photooxidative conditions. This method is of interest in green chemistry because of the use of molecular oxygen and visible light irradiation.References
- S. B. Mhaske and N. P. Argade, Tetrahedron, 2006, 62, 9787 CrossRef CAS PubMed.
- P. S. Reddy, P. P. Reddy and T. Vasantha, Heterocycles, 2003, 60, 183 CrossRef CAS.
- (a) K. Juvale, J. Gallus and M. Wiese, Bioorg. Med. Chem., 2013, 21, 7858 CrossRef CAS PubMed; (b) B. A. Bhat and D. P. Sahu, Synth. Commun., 2004, 34, 2169 CrossRef CAS PubMed; (c) M. J. Mphahlele, H. K. Paumo, A. M. El-Nahas and M. M. El-Hendawy, Molecules, 2014, 19, 795 CrossRef PubMed; (d) K. Q. Shawakfeh, Z. N. Ishtaiwi and N. H. Al-Said, Tetrahedron Lett., 2014, 55, 1329 CrossRef CAS PubMed; (e) X.-S. Wang, K. Yang, M.-M. Zhang and C.-S. Yao, Synth. Commun., 2010, 40, 2633 CrossRef CAS PubMed; (f) M. Bakavoli, A. Shiri, Z. Ebrahimpour and M. Rahimizadeh, Chin. Chem. Lett., 2008, 19, 1403 CrossRef CAS PubMed; (g) K. Juvale and M. Wiese, Bioorg. Med. Chem. Lett., 2012, 22, 6766 CrossRef CAS PubMed; (h) Z. Wang, M. Wang, X. Yao, Y. Li, J. Tan, L. Wang, W. Qiao, Y. Geng, Y. Liu and Q. Wang, Eur. J. Med. Chem., 2012, 53, 275 CrossRef CAS PubMed.
- (a) Y. Mitobe, S. Ito, T. Mizutani, T. Nagase, N. Sato and S. Tokita, Bioorg. Med. Chem. Lett., 2009, 19, 4075 CrossRef CAS PubMed; (b) J. Aw, Q. Shao, Y. Yang, T. Jiang, C. Ang and B. Xing, Chem.–Asian J., 2010, 5, 1317 CAS; (c) O. Thorn-Seshold, M. Vargas-Sanchez, S. McKeon and J. Hasserodt, Chem. Commun., 2012, 48, 6253 RSC; (d) M. Waibel and J. Hasserodt, Tetrahedron Lett., 2009, 50, 2767 CrossRef CAS PubMed.
- (a) R. J. Abdel-Jalil, H. M. Aldoqum, M. T. Ayoub and W. Voelter, Heterocycles, 2005, 65, 2061 CrossRef CAS; (b) A. Davoodnia, S. Allameh, A. R. Fakhari and N. Tavakoli-Hoseini, Chin. Chem. Lett., 2010, 21, 550 CrossRef CAS PubMed; (c) N. Montazeri, K. Pourshamsian, S. Yosefiyan and S. S. Momeni, J. Chem. Sci., 2012, 124, 883 CrossRef CAS; (d) R. J. Abdel-Jalil, W. Voelter and M. Saeed, Tetrahedron Lett., 2004, 45, 3475 CrossRef CAS PubMed.
- W. Xu, Y. Jin, H. Liu, Y. Jiang and H. Fu, Org. Lett., 2011, 13, 1274 CrossRef CAS PubMed.
- C. Balakumar, P. Lamba, D. P. Kishore, B. L. Narayana, K. V. Rao, K. Rajwinder, A. R. Rao and B. Shireesha, Eur. J. Med. Chem., 2010, 45, 4904 CrossRef CAS PubMed.
- T. Hisano, M. Ichikawa, A. Nakagawa and M. Tsuji, Chem. Pharm. Bull., 1975, 23, 1910 CrossRef CAS.
- W. Guan-Wu, M. Chun-Bao and K. Hui, Bull. Chem. Soc. Jpn., 2006, 79, 1426 CrossRef.
- T. G. Deligeorgiev, S. Kaloyanova, A. Vasilev, J.-J. Vaquero, J. Alvarez-Builla and A. M. Cuadro, Color. Technol., 2010, 126, 24 CAS.
- (a) H. Togo and S. Iida, Synlett, 2006, 2159 CrossRef CAS; (b) T. Nobuta, S. Hirashima, N. Tada, T. Miura and A. Itoh, Org. Lett., 2011, 13, 2576 CrossRef CAS PubMed; (c) T. Nobuta, A. Fujiya, N. Tada, T. Miura and A. Itoh, Synlett, 2012, 23, 2975 CrossRef CAS.
- M. Gao, Y. Yang, Y.-D. Wu, C. Deng, W.-M. Shu, D.-X. Zhang, L.-P. Cao, N.-F. She and A.-X. Wu, Org. Lett., 2010, 12, 4026 CrossRef CAS PubMed.
- (a) M. Uyanik, H. Okamoto, T. Yasui and K. Ishihara, Science, 2010, 328, 1376 CrossRef CAS PubMed; (b) M. Uyanik, D. Suzuki, T. Yasui and K. Ishihara, Angew. Chem., Int. Ed., 2011, 50, 5331 CrossRef CAS PubMed; (c) Z. Liu, J. Zhang, S. Chen, E. Shi, Y. Xu and X. Wan, Angew. Chem., Int. Ed., 2012, 51, 3231 CrossRef CAS PubMed; (d) J. Xie, H. Jiang, Y. Cheng and C. Zhu, Chem. Commun., 2012, 48, 979 RSC; (e) L. Chen, E. Shi, Z. J. Liu, S. Chen, W. Wei, H. Li, K. Xu and X. B. Wan, Chem.–Eur. J., 2011, 17, 4085 CrossRef CAS PubMed; (f) L. Ma, X. Wang, W. Yu and B. Han, Chem. Commun., 2011, 47, 11333 RSC; (g) W. Wei, C. Zhang, Y. Xu and X. Wan, Chem. Commun., 2011, 47, 10827 RSC; (h) T. Froehr, C. P. Sindlinger, U. Kloeckner, P. Finkbeiner and B. J. Nachtsheim, Org. Lett., 2011, 13, 3754 CrossRef CAS PubMed.
- (a) Y. Nagasawa, Y. Matsusaki, T. Hotta, T. Nobuta, N. Tada, T. Miura and A. Itoh, Tetrahedron Lett., 2014, 55, 6543 CrossRef CAS PubMed; (b) N. Tada, T. Ishigami, L. Cui, K. Ban, T. Miura and A. Itoh, Tetrahedron Lett., 2013, 54, 256 CrossRef CAS PubMed; (c) N. Tada, M. Shomura, L. Cui, T. Nobuta, T. Miura and A. Itoh, Synlett, 2011, 2896 CAS; (d) N. Tada, M. Shomura, H. Nakayama, T. Miura and A. Itoh, Synlett, 2010, 1979 CAS; (e) N. Kanai, H. Nakayama, N. Tada and A. Itoh, Org. Lett., 2010, 12, 1948 CrossRef CAS PubMed; (f) T. Nobuta, A. Fujiya, T. Yamaguchi, N. Tada, T. Miura and A. Itoh, RSC Adv., 2013, 3, 10189 RSC; (g) A. Fujiya, A. Kariya, T. Nobuta, N. Tada, T. Miura and A. Itoh, Synlett, 2014, 25, 884 CrossRef CAS.
- (a) N. Y. Kim and C.-H. Cheon, Tetrahedron Lett., 2014, 55, 2340 CrossRef CAS PubMed; (b) X. Chen, T. Chen, Y. Zhou, D. Han, L.-B. Han and S.-F. Yin, Org. Biomol. Chem., 2014, 12, 3802 RSC.
- (a) M. Uyanik, H. Hayashi and K. Ishihara, Science, 2014, 345, 291 CrossRef CAS PubMed; (b) Y. Tachikawa, Y. Nagasawa, S. Furuhashi, L. Cui, E. Yamaguchi, N. Tada, T. Miura and A. Itoh, RSC Adv., 2015, 5, 9591 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra07275a |
| This journal is © The Royal Society of Chemistry 2015 |