Influence of bovine serum albumin coated poly(lactic-co-glycolic acid) particles on differentiation of mesenchymal stem cells
Abstract
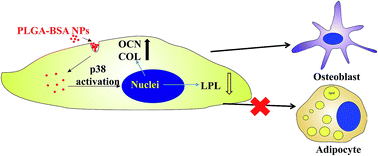
Interaction with colloidal particles may change the structure and function of the cytoskeleton, and influence cell shape and signal pathways, and thereby modulate the differentiation of stem cells. In this study, bovine serum albumin-coated poly(lactic-co-glycolic acid) particles (PLGA–BSA) were prepared and incubated with rat mesenchymal stem cells (MSCs). It was found that they promote osteogenic ALP activity and the expression of collagen type I (COL) and osteocalcin (OCN) of MSCs, but inhibit the expression of adipogenic peroxisome proliferator-activated receptor-gamma (PPARγ) and lipoprotein lipase (LPL) at both mRNA and protein levels. The p38 pathway is altered in the presence of PLGA–BSA particles, which might be responsible for the particle-induced differentiation of MSCs.

 Please wait while we load your content...
Please wait while we load your content...