Preparation and characterization of a novel porous Ti/SnO2–Sb2O3–CNT/PbO2 electrode for the anodic oxidation of phenol wastewater
Juntao Xinga,
Donghui Chena,
Wei Zhao*ab,
Xiaoling Penga,
Zilong Baia,
Wenwen Zhanga and
Xiuxian Zhaoa
aSchool of Chemical and Environmental Engineering, Shanghai Institute of Technology, Shanghai 201418, China. E-mail: zhaowei@sit.edu.cn; Tel: +86 13585633792
bCollege of Environmental Science and Engineering, Donghua University, Shanghai 201620, China
First published on 26th May 2015
Abstract
Porous Ti/SnO2–Sb2O3–CNT/PbO2 electrodes were successfully fabricated using a thermal decomposition technique and electro-deposition technologies. Characterization experiments including scanning electron microscopy (SEM), energy-dispersive spectroscopy (EDS), X-ray diffraction (XRD), cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS) and an accelerated lifetime test were performed to evaluate the effect of the CNT-doped SnO2–Sb2O3 intermediate layer on the PbO2 electrode. The results showed that CNT could be doped into the SnO2–Sb2O3 intermediate layer by thermal decomposition. Compared with the porous Ti/SnO2–Sb2O3 substrate, CNT-doping induced the substrate surface to form a fibrous structure, which suggested that the porous Ti/SnO2–Sb2O3–CNT substrate would provide more active sites for PbO2 deposition and could make a compact and fine surface coating. Moreover, the CNT-modified electrode had a higher active surface area and higher electrochemical activity than that without CNT doping. The life of the porous Ti/SnO2–Sb2O3–CNT/PbO2 electrode (296 h) was 1.38 times as much as that of the porous Ti/SnO2–Sb2O3/PbO2 electrode (214 h). The electro-catalytic oxidation of phenol in an aqueous solution was studied to evaluate the electrochemical oxidation ability in environmental applications. The porous Ti/SnO2–Sb2O3–CNT/PbO2 electrode displayed not only excellent electro-catalytic performance but also a low energy consumption using phenol as a model organic pollutant. The porous Ti/SnO2–Sb2O3–CNT/PbO2 electrode has a higher kinetic rate constant and chemical oxygen demand (COD), which is 1.73 and 1.09 times those of the porous Ti/SnO2–Sb2O3/PbO2 electrode, respectively. Moreover, CNT-doping can further increase the hydroxyl radical (˙OH) generation capacity. All these results illustrated that the porous Ti/SnO2–Sb2O3–CNT/PbO2 electrode could be used for pollutant degradation and had a great application potential.
1 Introduction
In recent years, the electrochemical oxidation of aqueous wastes containing non-biodegradable organics, such as phenol,1 lignin,2 4-chlorophenol,3 pentachlorophenol,4 and perfluorocarboxylic acids,5 has been extensively studied due to its many distinctive advantages,6 including environmental compatibility, versatile energy efficiency, low-volume application, and amenability to automation.6–8 For electrochemical oxidation, it has been found that the anode material plays a critical role in the degradation of organic pollutants.9 The reason is that electrode materials are conclusive for optimizing the electrochemical oxidation process because of their impact on the effectiveness of the mechanisms and reaction pathways.10 Hence, the current research hotspot is mainly focused on the development of stable anodes for the removal of persistent organic pollutants by electrochemical oxidation.7,11To date, literature results have shown that there are two main research focuses of electrochemical oxidation. The first research focus is the exploration of novel anode materials. The nature of the electrode material strongly affects both process selectivity and efficiency. In particular, anodes with low oxygen evolution overpotential, such as graphite,12 RuO2,13 and Pt,14 permit only the partial oxidation of organics, while anodes with high oxygen evolution overpotential, such as SnO2,15 PbO2,16 and BDD,17 favor high potential for the anodic oxidation of organic compounds. Among these, BDD anodes show the highest removal rate and stability, but their high cost and especially the difficulties of finding an appropriate substrate for the deposition of the diamond layer limit their large-scale application. The second research focus is to dope some metal elements into the oxide layer or intermediate layer, which can enhance the electro-catalytic activity and chemical or mechanical stability of oxide electrodes,18,19 such as doping with rare earth (Ce, Pr, La, etc.)20–23 or some metals (Au, Fe, Bi, etc.).24–26 Although the abovementioned methods show high effectiveness for the electro-catalytic oxidation of organic pollution, some problems still restrict their practical application such as short service life and complex preparation process.
In order to gain more reaction active sites and more contact between the coating catalyst and reactant to promote electro-catalytic efficiency, we introduced a porous Ti substrate and carbon nanotubes (CNTs) into an SnO2–Sb2O3 intermediate layer to prepare a novel porous Ti/SnO2–Sb2O3–CNT/PbO2 electrode. As a novel titanium matrix, porous Ti has the advantages of good corrosion resistance, high porosity, large surface area, and good biocompatibility, and is thus widely used in aerospace, and in the medical and chemical fields.27,28 Recent studies have revealed the significance of porous Ti for improving the performance of electrodes. Zhao et al. focused on systematically studying the effect of a porous Ti substrate on the surface structure and electrochemical properties of lead dioxide electrodes.28 Zhang et al. found that the PbO2/AC asymmetric electrochemical capacitor (AEC) based on porous-Ti/PbO2 has a higher energy density over traditional electrochemical capacitors and a longer lifespan over traditional lead acid batteries.29 In addition, carbon nanotubes (CNT), discovered by Iijima in 1991, are a novel material with good electrical properties and high chemical stability.30 CNTs have been proposed as the ideal metal catalyst support for sensing and electro-catalytic applications.15,31–33 The literature has mainly focused on CNT doped into the oxide layer to improve the catalytic ability of electrodes by an electro-deposition method;34 however, slight attention has been paid to CNT-doping into the intermediate layer of electrode by other methods. It is also expected that the CNT-doping will not only improve the catalytic performance of electrodes, but also increase the specific surface area and enhance the number of contacts to increase an electrode's service life.
In this study, we propose a novel modification method for the PbO2 electrode with the aim of improving the performance of the PbO2 electrode. To the best of our knowledge, this is the first time that CNTs have been introduced into a SnO2–Sb2O3 intermediate layer by thermal deposition. In addition, a systematic study was carried out to investigate the preparation and characterization of porous Ti/SnO2–Sb2O3–CNT/PbO2 compared with porous Ti/SnO2–Sb2O3/PbO2 without CNT-doping. The modified electrode was characterized, including its morphology, crystalline structure, electrochemical performance and accelerated service life. The oxidation performance and utilization of hydroxyl radicals on the electrodes were also evaluated. Phenol, as one of the most widely used pollutants in industrial wastewater, was selected as the target pollutant.
2 Experimental
2.1 Materials
The CNTs were purchased from Chengdu Organic Chemicals Co., Ltd. (China) with an outer diameter of 50 nm, and length of 10–20 μm. Porous titanium (purity 99.9%, 20 mm × 10 mm × 1 mm) was purchased from Baoji Jinkai Industrial Technology Co., Ltd. All the chemicals used in the experiments were received without further purification. All the solutions used in this work were prepared with deionized water.2.2 Electrode preparation
The treated titanium substrate was dipped in the solution for 5 min, and then dried at about 130 °C for 10 min, with the excess solvent being evaporated by hot air. Then, the treated titanium substrate was calcined at 500 °C for 15 min in a muffle furnace. All the above-mentioned processes were repeated twelve times. Finally, the electrodes were annealed at 500 °C for 60 min.
More details concerning the electrode preparation are given in our previous study.35
2.3 Electrode characterization
All the electrochemical experiments were carried out at room temperature (25 ± 2 °C). All the solutions were prepared with deionized water, and the all reagents used in the experiments were of analytic grade.
2.4 Electrochemical degradation tests
Phenol was selected as a sample of aromatic contamination for studying the CNT-modified electrodes. The electrochemical degradation was carried out in a cylindrical single compartment cell equipped with a magnetic stirrer and a jacketed cooler to maintain a constant temperature. The fabricated PbO2 electrode was used as an anode and stainless copper with the same dimension was used as the cathode with a distance of 1.5 cm between the two electrodes.The current density was controlled to be a constant 30 mA cm−2 by a direct current power supply (RXN-605D, China). The stirring rate was about 800 rpm. The experiments were carried out at 25 °C for 300 min. The volume of phenol solution was 80 mL with an initial concentration of 100 mg L−1, and 0.1 mol L−1 Na2SO4 was added to the solution. During the experiments, liquid samples were withdrawn from the electrolytic cell at fixed time intervals to determine the variation of phenol concentration and COD and to evaluate the reaction kinetics. The concentration of phenol was analyzed using a Varian high performance liquid-chromatography (HPLC) with a UV detector set at 270 nm. A C-18 column was used to separate the organics, while the mobile phase made of 30% CH3CN and 70% water was at a flow rate of 1 mL min−1. COD was digested by potassium dichromate using a 5B-1F Speed Digester (Lianhua Tech Co., China) at 150 °C for 10 min and determined by ultraviolet spectrophotometry at 610 nm using a TU-1810 UV/visible analysis spectrophotometer (Beijing Puxi Instrument Co. Ltd.). Each experiment was conducted three times in order to keep the relative standard deviations (RSD) less than 5%.
The general current efficiency (GCE), representing an average value of current efficiency between the initial time t = 0 and t, was calculated based on the results of the COD test and the following equation:7
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485 C mol−1); V is the volume of the solution (L); and 8 is the value of the oxygen equivalent mass (g eq.−1).
485 C mol−1); V is the volume of the solution (L); and 8 is the value of the oxygen equivalent mass (g eq.−1).
The specific energy consumption (SEC) was the energy consumption for the removal of one kg of COD and was calculated as follows (expressed in kW h):8
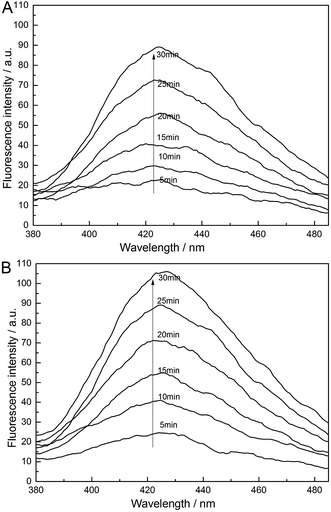
In addition, we used the fluorescence method based on terephthalic acid, as this is a well-known ˙OH scavenger that can be used for estimating the amount of ˙OH radicals generated under various conditions by a fluorescence spectrophotometer (Perkin-Elmer LS-50, American). An aqueous solution of a 200 mL volume containing 0.5 mol L−1 terephthalic acid, 0.5 g L−1 NaOH and 0.25 mol L−1 Na2SO4 was used as the electrolyte solution. The anode was a prepared PbO2 electrode and the cathode was a stainless steel sheet. Hydroxyl radical production was performed at a current density of 30 mA cm−2 at 30 °C. During the experiments, samples were drawn from the reactor every 5 min and diluted 10 times with deionized water, then analyzed with a fluorescence spectrophotometer. The fluorescence spectra were recorded in the range of 380 to 520 nm, using a 315 nm excitation wavelength.
3 Results and discussion
3.1 Surface morphology and crystal structure
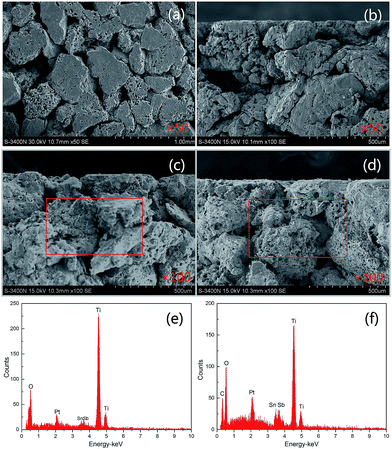
Fig. 2(a)–(d) shows the morphologies of the interlayer and surface layer of different electrodes. It is clearly seen from Fig. 2(a) and (b) that the introduction of CNT-doping into the SnO2–Sb2O3 interlayer film significantly affected the film morphology. In contrast with the porous Ti/SnO2–Sb2O3 substrate, CNT-doping induced the substrate surface to form a fibrous structure; hence, porous Ti/SnO2–Sb2O3–CNT had a larger specific surface area, which could provide more active sites for electro-catalytic oxidation. In addition, the fibrous structure can make the PbO2 to deposit more firmly and tightly. The CNT-modified electrode was expected to have better electrochemical properties and physico-chemical properties, which was further proved by the following experiments. To further check the impact of CNT-doping on the formation of PbO2, Fig. 2(c) and (d) display the electrode crystal structure and appearance of porous Ti/SnO2–Sb2O3/PbO2 and porous Ti/SnO2–Sb2O3–CNT/PbO2 with a magnification of 100× and 4000× in the insets, respectively. It is noticeable that the particles sizes of porous Ti/SnO2–Sb2O3–CNT/PbO2 become smaller than those of porous Ti/SnO2–Sb2O3/PbO2, and the coating particles grown on the SnO2–Sb2O3–CNT intermediate layer did not present fissures or laminations and appeared to be more compact and uniformly distributed.
Fig. 2(g) and (h) show the EDS spectrum of the coating of porous Ti/SnO2–Sb2O3 and porous Ti/SnO2–Sb2O3–CNT, respectively (Pt peak comes from the platinum coating for SEM). Sn, Sb, Ti and O were clearly observed, indicating the formation of the oxide mixture of Sn and Sb. Due to porous Ti with larger surface areas, the oxide layer may not completely cover porous Ti substrate surface. The C peak was detected in EDS (Fig. 2(h)), showing that CNT was successfully doped into the middle layer (SnO2–Sb2O3) by thermal decomposition method and not oxidized during the calcination process. This result was not consistent with the result reported in literature.15 Based on the results from the SEM and EDS analyses, we conclude that the CNT was successfully doped into SnO2–Sb2O3 intermediate layer by calcination process and would be beneficial to electrochemical properties. Based on the results from the abovementioned SEM-EDS analyses, the formation process of porous Ti/SnO2–Sb2O3–CNT is described in Fig. 3. In addition, we use the gravimetric method to calculate the growing rate of SnO2–Sb2O3–CNT/PbO2 and the value is 1.979 mg cm−2.
3.2 Electrochemical characterization of the electrodes
| (q*)−1 = (q*T)−1 + kv1/2 | (1) |
The total charge quantity q*T stands for the quantity of theoretically electrochemical active sites of the electrode surface, and v stands for the scan rate of voltage, while k is a constant. In order to determine the electrochemical active surface areas of porous Ti/SnO2–Sb2O3/PbO2 and porous Ti/SnO2–Sb2O3–CNT/PbO2, q* was tested in Na2SO4 solution. Special adsorption occurs between sulfate ions and PbO2, forming insoluble PbSO4, which results in pseudo-capacitance (eqn (2) and (3)):
| H+ + SO4ads2− ⇔ HSO4ads− | (2) |
| 4HSO4ads− + PbO2 + 2e− = PbSO4ads + 3SO4ads2− + H2O | (3) |
Therefore, the q* in the pseudo-capacitance region of PbO2 in Na2SO4 solution can be defined as the electrochemical active surface area of PbO2 in the sulfate electrolyte. Fig. 5(A) shows the cyclic voltammograms of porous Ti/SnO2–Sb2O3/PbO2 and porous Ti/SnO2–Sb2O3–CNT/PbO2, q* was integrated from the cyclic voltammetric curves over the whole potential range from 0.3 V to 0.8 V. The relationship of the reciprocal of q* versus the square root of the scan rate is shown in Fig. 5(B). For PbO2 electrodes, the q* at low scan rate reflects the deep discharge performance; the q* at high scan rate reflects the power performance. The q* of PbO2 electrode at a scan rate of 10 and 50 mV s−1 are listed in Table 1. The k constant is the slope of our straight lines in Fig. 5(B). k constant represents the change rate of the electrochemical active surface area with increasing scan rate. The smaller the k constant is, the slower the electrochemical active surface area declines with increasing the scan rate. Therefore, comparing with the value of q* and k, the result indicated that the porous Ti/SnO2–Sb2O3–CNT/PbO2 electrode had the highest active surface area.
| Electrode | k constant | q* at 10 mV s−1 | q* at 50 mV s−1 |
|---|---|---|---|
| Porous Ti/SnO2–Sb2O3/PbO2 | 33.5588 | 0.0093 | 0.0042 |
| Porous Ti/SnO2–Sb2O3–CNT/PbO2 | 14.3733 | 0.0186 | 0.0091 |
| PbO2 + H2SO4 + 2H+ + 2e− ⇔ PbSO4 + 2H2O | (4) |
 | ||
| Fig. 6 Cyclic voltammogram curves of different electrodes: (a) porous Ti/SnO2–Sb2O3/PbO2 and (b) porous Ti/SnO2–Sb2O3–CNT/PbO2 in 0.5 mol L−1 H2SO4 solution at a scan rate of 50 mV s−1. | ||
In addition, The CNT-modified electrode presented higher oxidation–reduction peak currents than the other electrode without CNT-doping, meaning that many more substances are involved in the oxidation reaction. The reason for this mainly lies in the porous Ti/SnO2–Sb2O3–CNT/PbO2 electrode having a larger active surface area, which provides more active site for the electrochemical reaction. The result also further proved the above conclusion. In conclusion, the introduction of a CNT-doped interlayer is expected to increase the oxidation ability and catalytic activity of porous Ti/SnO2–Sb2O3/PbO2.
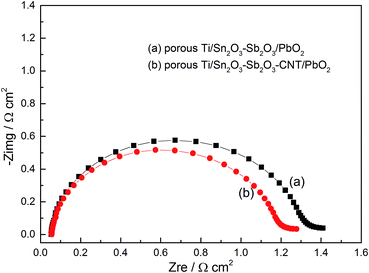 | ||
| Fig. 7 EIS plots in the 0.5 M acidic solution: (a) porous Ti/SnO2–Sb2O3/PbO2 electrode and (b) porous Ti/SnO2–Sb2O3–CNT/PbO2 electrode. Electrode potential: 1.8 V vs. SCE. | ||
| Electrode | Rs/Ω cm2 | Qdl/Ω cm−2 sn | Rct/Ω/Ω cm2 | n |
|---|---|---|---|---|
| Porous Ti/SnO2–Sb2O3/PbO2 | 0.3962 | 0.0067 | 1.315 | 0.9591 |
| Porous Ti/SnO2–Sb2O3–CNT/PbO2 | 0.2847 | 0.0079 | 1.187 | 0.9531 |
Two evident semicircles appear in the electrochemical impedance spectra in Fig. 7. The diameter of the semicircle size reflects Rct and the resistance values were 1.315 Ω cm2 and 1.187 Ω cm2 in Table 2, respectively, indicating that the oxygen evolution reaction activity of the porous Ti/SnO2–Sb2O3–CNT/PbO2 electrode was slightly higher. According to the reaction mechanism of electrode oxygen evolution, the oxygen evolution activity depends on the active sites of active coating, whereby the more the active sites, the greater the reaction activity. Hence CNT-doping into the intermediate layer also increases the active sites, which can provide a larger specific surface area. The result was also further proven by the Rs values in Table 2 showing that the porous Ti/SnO2–Sb2O3–CNT/PbO2 electrode prepared had the smallest Rs, indicating the largest electrochemical active surface area. From the abovementioned results, it can be concluded that CNT-doping can reduce the resistance of the active material.
3.3 Electrochemical degradation test
In addition, the processes of degradation were found to well fit the pseudo-first-order model for all electrodes as shown in eqn (5). The fitting results are shown in Fig. 10(B).
 | (5) |
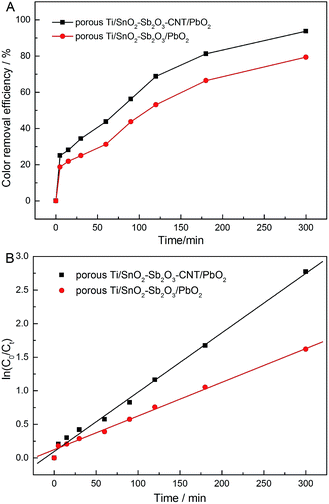
The COD reduction is very important to monitor the performance of the different electrodes. Hence, COD removals of phenol by the two different electrodes were compared to further evaluate the effect of the CNT-doping for the electro-catalytic oxidation. The variations of COD with electrolysis time are shown in Fig. 11. Clearly, the COD was removed more rapidly on the porous Ti/SnO2–Sb2O3–CNT/PbO2 than that on the porous Ti/SnO2–Sb2O3/PbO2. About 95% and 87% of COD removal were achieved in 300 min, respectively. Hence, the organic compounds were more effectively degraded and oxidized on the porous Ti/SnO2–Sb2O3–CNT/PbO2 electrode. The high phenol removal rate can be ascribed to the larger active surface area.22 In addition, the general current efficiency (GCE) and specific energy consumption (SEC) were also calculated and are listed in Table 3.
| Electrode sample | GCE | SEC/kW h (kg COD)−1 |
|---|---|---|
| Porous Ti/SnO2–Sb2O3/PbO2 | 61.3% | 289.45 |
| Porous Ti/SnO2–Sb2O3–CNT/PbO2 | 67.1% | 250.62 |
Evidently, all the above-mentioned results indicate that CNT can effectively enhance the surface area of the electrode, which improves the performance of the electrode. This agrees well with the experimental results presented above.
4 Conclusions
A highly effective porous Ti/SnO2–Sb2O3–CNT/PbO2 electrode was successfully prepared on a porous titanium substrate by thermal decomposition and electro-deposition. The electrode modified with CNT versus without CNT has a higher specific surface area, which can improve the interlayer coating structure effectively and favor the formation of PbO2 during electro-deposition. The voltammetric charge quantity indicated that the electrode modified with CNT enhanced the mass transfer rate on a PbO2 electrode. The results of the accelerated life tests showed that the service life of the porous Ti/SnO2–Sb2O3–CNT/PbO2 electrode was longer than that of electrodes without CNT-doping, which was 1.35 times that of the porous Ti/SnO2–Sb2O3/PbO2 electrode. The electrode modified with CNT was then applied in phenol wastewater treatment and presented excellent ability for organic pollutant oxidation and degradation compared with the electrode without CNT modification. After 5 h, the phenol was almost completely decomposed using porous Ti/SnO2–Sb2O3–CNT/PbO2, and the COD removal reached 94.81%, while the value for porous Ti/SnO2–Sb2O3/PbO2 was just 87.01%. Moreover, the general current efficiency (GCE) and specific energy consumption (SCE) were superior to that of the porous Ti/SnO2–Sb2O3/PbO2 electrode (67.1%, 250.62 kg−1 COD−1 versus 61.3%, 289.45 kg−1 COD−1). In addition, CNT-doping can further increase the hydroxyl radical (˙OH) generation capacity. Considering the improved electro-catalytic oxidation performance and service life in practice, the porous Ti/SnO2–Sb2O3–CNT/PbO2 anodes can be prepared on a large scale and applied in industry.Acknowledgements
The authors are grateful for the financial support provided by the Innovative Program of Activities for University in Shanghai (no. DCX2015064). Wenli Zhang (Jilin University) and Quansheng Zhang (Shanghai Institute of Technology) are also gratefully acknowledged for supplying experimental guidance and the electrochemical workstation, respectively.References
- H. Li, Y. Chen, Y. Zhang, W. Han, X. Sun, J. Li and L. Wang, J. Electroanal. Chem., 2013, 689, 193–200 CrossRef CAS PubMed.
- D. Shao, J. Liang, X. Cui, H. Xu and W. Yan, Chem. Eng. J., 2014, 244, 288–295 CrossRef CAS PubMed.
- X. Duan, L. Tian, W. Liu and L. Chang, Electrochim. Acta, 2013, 94, 192–197 CrossRef CAS PubMed.
- J. Niu, Y. Bao, Y. Li and Z. Chai, Chemosphere, 2013, 92, 1571–1577 CrossRef CAS PubMed.
- J. Niu, H. Lin, J. Xu, H. Wu and Y. Li, Environ. Sci. Technol., 2013, 47, 4959 CrossRef CAS.
- E. Brillas and C. A. Martínez-Huitle, Appl. Catal., B, 2015, 166–167, 603–643 CrossRef CAS PubMed.
- M. Panizza and G. Cerisola, Chem. Rev., 2009, 109, 6541–6569 CrossRef CAS PubMed.
- C. Martínez-Huitle and S. Ferro, Chem. Soc. Rev., 2006, 35, 1324 RSC.
- A. B. Velichenko, R. Amadelli, E. A. Baranova, D. V. Girenko and F. I. Danilov, J. Electroanal. Chem., 2002, 527, 56–64 CrossRef CAS.
- Y. Xia, Q. Dai and J. Chen, J. Electroanal. Chem., 2015, 744, 117–125 CrossRef CAS PubMed.
- G. Zhao, Y. Zhang, Y. Lei, B. Lv, J. Gao, Y. Zhang and D. Li, Environ. Sci. Technol., 2010, 44, 1754–1759 CrossRef CAS PubMed.
- Y. Shen, H. Xu, P. Xu, X. Wu, Y. Dong and L. Lu, Electrochim. Acta, 2014, 132, 37–41 CrossRef CAS PubMed.
- L. Du, J. Wu and C. Hu, Electrochim. Acta, 2012, 68, 69–73 CrossRef CAS PubMed.
- A. Fernandes, D. Santos, M. J. Pacheco, L. Ciríaco and A. Lopes, Appl. Catal., B, 2014, 148–149, 288–294 CrossRef CAS PubMed.
- L. Zhang, L. Xu, J. He and J. Zhang, Electrochim. Acta, 2014, 117, 192–201 CrossRef CAS PubMed.
- J. P. Carr and N. A. Hampson, Chem. Rev., 1972, 72, 679–703 CrossRef CAS.
- M. Panizza, A. Kapalka and C. Comninellis, Electrochim. Acta, 2008, 53, 2289–2295 CrossRef CAS PubMed.
- X. Hao, L. Jingjing, Y. Wei and W. Chu, Rare Met. Mater. Eng., 2013, 42, 885–890 CrossRef.
- A. B. Velichenko and D. Devilliers, J. Fluorine Chem., 2007, 128, 269–276 CrossRef CAS PubMed.
- Y. Feng, Y. Cui, B. Logan and Z. Liu, Chemosphere, 2008, 70, 1629–1636 CrossRef CAS PubMed.
- Y. Feng, Y.-H. Cui, J. Liu and B. E. Logan, J. Hazard. Mater., 2010, 178, 29–34 CrossRef CAS PubMed.
- H. Xu, A. Li and X. Cheng, Int. J. Electrochem. Sci., 2011, 6, 5114–5124 CAS.
- H. Lin, J. Niu, J. Xu, Y. Li and Y. Pan, Electrochim. Acta, 2013, 97, 167–174 CrossRef CAS PubMed.
- R. Berenguer, C. Quijada and E. Morallón, Electrochim. Acta, 2009, 54, 5230–5238 CrossRef CAS PubMed.
- A. Moncada, S. Piazza, C. Sunseri and R. Inguanta, J. Power Sources, 2015, 275, 181–188 CrossRef CAS PubMed.
- Y. Song, G. Wei and R. Xiong, Electrochim. Acta, 2007, 52, 7022–7027 CrossRef CAS PubMed.
- M. Manjaiah, S. Narendranath and S. Basavarajappa, Trans. Nonferrous Met. Soc. China, 2014, 24, 12–21 CrossRef CAS.
- W. Zhao, J. Xing, D. Chen, Z. Bai and Y. Xia, RSC Adv., 2015, 5, 26530–26539 RSC.
- W. Zhang, H. Lin, H. Kong, H. Lu, Z. Yang and T. Liu, Int. J. Hydrogen Energy, 2014, 39, 17153–17161 CrossRef CAS PubMed.
- S. Iijima, Nature, 1991, 354, 56–58 CrossRef CAS PubMed.
- F. Hu, Z. Dong, X. Cui and W. Chen, Electrochim. Acta, 2011, 56, 1576–1580 CrossRef CAS PubMed.
- D. Yang, L. Zhu and X. Jiang, J. Electroanal. Chem., 2010, 640, 17–22 CrossRef CAS PubMed.
- F. Hu, X. Cui and W. Chen, Electrochem. Solid-State Lett., 2010, 13, F20 CrossRef CAS PubMed.
- X. Duan, F. Ma, Z. Yuan, L. Chang and X. Jin, J. Electroanal. Chem., 2012, 677–680, 90–100 CrossRef CAS PubMed.
- W. Zhao, J. Xing, D. Chen, Z. Bai and Y. Xia, RSC Adv., 2015, 5, 26530–26539 RSC.
- H. Vogt, Electrochim. Acta, 1994, 39, 1981–1983 CrossRef CAS.
- S. Trasatti, Electrochim. Acta, 1991, 36, 1659–1667 CrossRef CAS.
- Y. Chen, L. Hong, H. Xue, W. Han, L. Wang, X. Sun and J. Li, J. Electroanal. Chem., 2010, 648, 119–127 CrossRef CAS PubMed.
- D. Pavlov, A. Kirchev, M. Stoycheva and B. Monahov, J. Power Sources, 2004, 137, 288–308 CrossRef CAS PubMed.
- J. Zhao, C. Zhu, J. Lu, C. Hu, S. Peng and T. Chen, Electrochim. Acta, 2014, 118, 169–175 CrossRef CAS PubMed.
- X. Zhu, J. Ni, H. Li, Y. Jiang, X. Xing and A. G. L. Borthwick, Electrochim. Acta, 2010, 55, 5569–5575 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |