Evaluation of methionine and tryptophan derivatised vehicles: Met-ac-TE3A/Trp-ac-TE3A for tumor imaging†
Sweta Singhab,
Anjani K. Tiwari*a,
Raunak Varshneya,
Rashi Mathura,
Puja P. Hazaria,
B. Singhb and
Anil K. Mishra*a
aDivision of Cyclotron and Radiopharmaceutical Sciences, Institute of Nuclear Medicine and Allied Sciences, Brig. S. K. Mazumdar Road, Timarpur, Delhi-110054, India. E-mail: akmishra63@gmail.com; dcrs_inmas@rediffmail.com; Fax: +91 1123919509; Tel: +91 23905117
bDepartment of Chemistry, Banaras Hindu University, Varanasi-221005, India
First published on 24th April 2015
Abstract
Two novel amino acid (methionine and tryptophan) appended 1,4,8,11-tetraazacyclotetradecane triacetate (TE3A) compounds Met-ac-TE3A and Trp-ac-TE3A were synthesized and evaluated for imaging applications. The pharmacokinetics of these compounds was analyzed by 99mTc labeled tracer methods. In vitro human serum stability of 99mTc labeled Met-ac-TE3A/Trp-ac-TE3A was found to be 96.5% and 96.0% after 24 h respectively. Blood kinetics of both the labeled probes on normal rabbits showed biphasic clearance. The tumor (EAT cell line) grafted in balb/c mice were readily identifiable in the gamma images. Biodistribution revealed significant tumor uptake and good contrast in the EAT tumor bearing mice and also showed high tumor/muscles ratio which is a requisite condition to work as SPECT-radiopharmaceutical for tumor imaging. To look its future applicability for therapy using M+2 and M+3 metal ions, we performed thermodynamic stability constants of complexes derived from Met-ac-TE3A and Trp-ac-TE3A with CuII and LnIII metal ions.
1. Introduction
Radiotracer based imaging probes are essential for the diagnosis of disease, monitoring and targeted therapy in the field of neuroimaging, oncology, neurooncology and infections using positron emission tomography (PET) and single photon emission computed tomography (SPECT). Radiolabeled amino acids has been proved a diverse and useful class of PET and SPECT tracers that records amino acid transportation and uptake exhibited by many tumor cells i.e. brain, neuroendocrine, prostate cancer.1–4 Increase rate for amino acid uptake is one of the earliest and most important events associated with proliferation.5Recent studies have shown that of IDO (indoleamine 2,3-dioxygenase) is overexpressed in a variety of human tumors, including lung tumors resulting increase of abnormal tryptophan metabolism via the kynurenine pathway. L-Tryptophan is a substrate of IDO.6–8 Intracellular IDO activity may result in the trapping of polar tryptophan (or its derivative) metabolites or indirect accumulation via intracellular L-tryptophan depletion. There are many evidences that show fast growing progression of tumors because of a failure of the immune system to maintain control over budding tumors. Various studies indicate that the consumption of tryptophan is critical factor in progressive tumor.9,10 The PET radiotracer, [11C] methyl-L-tryptophan (AMT) is well suited for such studies; AMT is not a substrate for protein synthesis11 but can be metabolized by IDO because of the low substrate specificity of this enzyme.12
Similarly the major metabolic function of methionine is in protein synthesis and conversion to S-adenosylmethionine (Adomet), which is required in multiple metabolic pathways. Methionine (Met) dependence has been shown in vitro in a number of human cell lines of different cellular origin, and the dependence may reflect the overall imbalance in transmethylation. Metabolic defects in cancer cells often manifest in the inability to grow in media where Met has been replaced by its precursor homocysteine.
It was therefore considered valuable to focus our attention on the development of specific amino acid based imaging system that can be utilized for imaging techniques viz. PET, SPECT and magnetic resonance imaging (MRI) for tumour targeting by conjugation of amino acid with a suitable chelate. Currently a variety of cyclic and acyclic polyamino-polypcarboxylates are being evaluated as vehicles for variety of radiopharmaceuticals and MR agents. These polyamino-polycarboxylates have capability to adopt an organized conformation in the complex formation with metal ions. Two of the most important chelators studied were DOTA and TETA.13 In continuation of our work for design of novel agent having these vehicles, we previously synthesised DTPA-bis(methionine)14 and DO3A-Act-Met15 for imaging applications.
Here, we report the synthesis of two new ligand, synthesis of (11-((3-(1H-indol-3-yl)-1-methoxy-oxopropan-2-yl)amino)-2-oxoethyl)-1,4,8,11-tetraazacyclo tetradecane-(1,4,8-triyl)triacetic acid, Trp-ac-TETA and 2,2,2,-(11-((3-(1H-indol-3-yl)-1-methoxy-oxopropan-2-yl)amino)-2-oxoethyl)-1,4,8,11-tetraazacyclotetradecane-(1,4,8-triyl)triacetic acid, Met-ac-TETA. After wet synthesis and spectroscopic characterisation both the molecules were evaluated in vitro and in vivo after coordinating with suitable metal ion of biomedical interest.
2. Experimental
2.1. Ligand synthesis and characterization
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, chloroform
1, chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol), the reaction mixture was cooled, filtered, and evaporated under reduced pressure to give crude oily residue. The compound was purified by column chromatography (methanol
methanol), the reaction mixture was cooled, filtered, and evaporated under reduced pressure to give crude oily residue. The compound was purified by column chromatography (methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform) to give the compounds.
chloroform) to give the compounds.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; dichloromethane
1; dichloromethane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol). After the completion of the reaction, the reaction mixture was filtered and evaporated to dryness. The crude compound was purified by column chromatography (silica gel, 9
methanol). After the completion of the reaction, the reaction mixture was filtered and evaporated to dryness. The crude compound was purified by column chromatography (silica gel, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 chloroform
1 chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol) to give 1 (t-Bu-TE3A) as white powder (2.03 g, 75%). 1H NMR (400 MHz, CDCl3, TMS): δH = 1.45 (3 s, 27H; C(CH3)3), 1.62–1.63 (m, 4H), 1.99 (brs, 1H), 2.55–3.40 (m, 22H); 13C-NMR (100 MHz, CDCl3): δC = 23.52, 24.37 (CH2), 29.01, 29.07, 29.12, (C(CH3), 47.06, 47.53, 49.86, 50.87, 51.88, 53.38, 55.59, 56.70, 56.89, 81.72, 82.15, 82.40 (C(CH3)3), 171.53, 172.47, 172.62 (CO). m/z (ESI) found 543.9 [M + H]+, C28H54N4O6, calculated 542.7.
methanol) to give 1 (t-Bu-TE3A) as white powder (2.03 g, 75%). 1H NMR (400 MHz, CDCl3, TMS): δH = 1.45 (3 s, 27H; C(CH3)3), 1.62–1.63 (m, 4H), 1.99 (brs, 1H), 2.55–3.40 (m, 22H); 13C-NMR (100 MHz, CDCl3): δC = 23.52, 24.37 (CH2), 29.01, 29.07, 29.12, (C(CH3), 47.06, 47.53, 49.86, 50.87, 51.88, 53.38, 55.59, 56.70, 56.89, 81.72, 82.15, 82.40 (C(CH3)3), 171.53, 172.47, 172.62 (CO). m/z (ESI) found 543.9 [M + H]+, C28H54N4O6, calculated 542.7.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 chloroform
5 chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol) to give the 4 (0.41 g, 75%) as a brown solid. 1HNMR (400 MHz, CDCl3, TMS): δH = 1.39 (s, 27H; C(CH3)3), 1.58–1.59 (m, 4H), 2.04–2.15 (m, 5H, SCH3 and methionine proton), 2.46–2.69 (m, 19H), 3.18 (s, 4H), 3.25 (s, 2H), 3.74 (s, 2H), 3.68 (s, 3H, OCH3), 4.58–4.63 (m, 1H); 13C-NMR (100 MHz, CDCl3): δC = 15.38 (CH3), 25.27, 25.65, 28.15 (C(CH3)3), 30.24, 31.20, 42.38, 50.12, 50.20, 50.52, 51.10, 51.47, 51.76, 52.25, 52.38, 53.00, 56.18, 56.31, 56.36, 58.55, 80.60, 80.65, 80.78 (C(CH3)3), 170.50, 170.83, 171.03, 172.10, 172.54, (CO). m/z (ESI) found 747.8 (M + H+), 769.7 (M + Na+), C36H67N5O9S, calculated 746.0.
methanol) to give the 4 (0.41 g, 75%) as a brown solid. 1HNMR (400 MHz, CDCl3, TMS): δH = 1.39 (s, 27H; C(CH3)3), 1.58–1.59 (m, 4H), 2.04–2.15 (m, 5H, SCH3 and methionine proton), 2.46–2.69 (m, 19H), 3.18 (s, 4H), 3.25 (s, 2H), 3.74 (s, 2H), 3.68 (s, 3H, OCH3), 4.58–4.63 (m, 1H); 13C-NMR (100 MHz, CDCl3): δC = 15.38 (CH3), 25.27, 25.65, 28.15 (C(CH3)3), 30.24, 31.20, 42.38, 50.12, 50.20, 50.52, 51.10, 51.47, 51.76, 52.25, 52.38, 53.00, 56.18, 56.31, 56.36, 58.55, 80.60, 80.65, 80.78 (C(CH3)3), 170.50, 170.83, 171.03, 172.10, 172.54, (CO). m/z (ESI) found 747.8 (M + H+), 769.7 (M + Na+), C36H67N5O9S, calculated 746.0.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 chloroform
5 chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol) to give the compound as a brown solid (0.56 g, 75%).1HNMR (400 MHz, CDCl3, TMS): δH = 1.44–1.47 (m, 31H), 2.42–2.65 (m, 15H), 2.99–3.24 (m, 11H), 3.68 (s, 3H; CH3–O–), 4.82–4.87 (m, 1H; CH–NH–), 7.08–7.52 (m, 5H; indolylprotons); 13CNMR, (100 MHz, CDCl3): δC = 25.12, 25.38, 28.20 (CH3)3, 42.41(OCH3), 50.81, 51.11, 51.17, 51.53, 51.71, 51.97, 52.23, 52.26, 52.49, 52.61, 52.75, 53.29, 55.84, 56.41, 80.75, 80.87, 80.96, (C(CH3)3), 109.38, 111.57, 118.38, 119.39, 121.98, 123.29, 127.44, 136.43, 165.97, 170.76 (CO), 171.14 (CO), 171.19 (CO), 172.38 (CO). m/z (ESI) found 802 (M + 2H+), C42H68N6O9, calculated 800.0.
methanol) to give the compound as a brown solid (0.56 g, 75%).1HNMR (400 MHz, CDCl3, TMS): δH = 1.44–1.47 (m, 31H), 2.42–2.65 (m, 15H), 2.99–3.24 (m, 11H), 3.68 (s, 3H; CH3–O–), 4.82–4.87 (m, 1H; CH–NH–), 7.08–7.52 (m, 5H; indolylprotons); 13CNMR, (100 MHz, CDCl3): δC = 25.12, 25.38, 28.20 (CH3)3, 42.41(OCH3), 50.81, 51.11, 51.17, 51.53, 51.71, 51.97, 52.23, 52.26, 52.49, 52.61, 52.75, 53.29, 55.84, 56.41, 80.75, 80.87, 80.96, (C(CH3)3), 109.38, 111.57, 118.38, 119.39, 121.98, 123.29, 127.44, 136.43, 165.97, 170.76 (CO), 171.14 (CO), 171.19 (CO), 172.38 (CO). m/z (ESI) found 802 (M + 2H+), C42H68N6O9, calculated 800.0.Chemical purity: >99% by HPLC: Capcell Pack UG80 C18 column, 4.6 mm i.d. × 250 mm; MeCN/H2O/Et3N, 75/25/0.01 (v/v/v); flow rate, 1.0 mL min−1; λuv, 254 nm); retention time (tR), 9.7 min (ESI Fig. 1†).
High resolution mass spectrometer (HRMS) (FAB) calculated for C30H44N6O9, 632.4132; observed: 632.3190.
Chemical purity: >99% by HPLC: Capcell Pack UG80 C18 column, 4.6 mm i.d. × 250 mm; MeCN/H2O/Et3N, 80/20/0.01 (v/v/v); flow rate, 1.0 mL min−1; λuv, 254 nm; retention time (tR), 8.1 min (ESI Fig. 2†).
2.2. Determination of protonation and stability constant
The protonation constant of [Trp-ac-TE3A] and [Met-ac-TE3A] and stability constants of the complexes formed with EuIII and CuII have been determined by pH potentiometric titrations (Table 1). Potentiometric measurements were carried out with an automatic titration system consisting of Metrohm 713 pH meter equipped with a Metrohm A.60262.100 electrode 800 Dosino autoburet. The acidity constants of both the compound were determined potentiometrically by titrating 25.0 mL of aqueous 2.5 mM HCl in the presence of 0.01 M both substituted TE3A with 0.1 M NMe4OH.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β ± SD, n = 3) of the ligands DTPA, DOTA and Trp-ac-TE3A/Met-ac-TE3A at 25 °C
β ± SD, n = 3) of the ligands DTPA, DOTA and Trp-ac-TE3A/Met-ac-TE3A at 25 °C
| Protonation constants | DTPA30a | DOTA30a | Trp-ac-TE3A | Met-ac-TE3A |
|---|---|---|---|---|
| 0.1 M KCl | 0.1 M KCl | 0.1 M KCl | 0.1 M KCl | |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K1H K1H |
10.48 | 11.14 | 10.40 | 10.25 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K2H K2H |
8.60 | 9.69 | 8.10 | 8.80 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K3H K3H |
4.28 | 4.84 | 4.10 | 4.00 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K4H K4H |
2.6 | 3.9 | 2.65 | 2.52 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K5H K5H |
2.0 | — | — |
2.3. Radiolabeling of Met-ac-TE3A/Trp-ac-TE3A with 99mTc
Met-ac-TE3A/Trp-ac-TE3A (1 mg) was dissolved in a shielded vial and stannous chloride (300 μL; 1 mg dissolved in N2 purged 1 mL of 10% acetic acid) was added followed by addition of freshly eluted (<1 h) 99mTechnetium pertechnetate (82 MBq; 200 μL). The pH of the reaction mixture was adjusted to 7 with 0.1 M Na2CO3 and purged with N2, and shaken to mix. The vial was allowed to stand for 15 min at room temperature (25 °C).2.4. Radiochemical purity
The number of ligand molecules involved in complexation with 99mTc was determined by ascending instant thin layer chromatography on ITLC-SG (Paul Gelman,USA) strips using 100% acetone as developing solvent and simultaneously in pyridine/acetic acid/water (PAW) (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) and saline. Each TLC was cut in 0.5 cm segments and counts of each segment were taken. By using this method percentage of free Na99mTcO4−, reduced/hydrolysed 99mTc, and the complex formed between 99mTc and Met-ac-TE3A/Trp-ac-TE3A conjugate could be calculated. Met-ac-TE3A/Trp-ac-TE3A conjugate remained at the origin, and free technetium traveled with the solvent front in acetone.
1.5) and saline. Each TLC was cut in 0.5 cm segments and counts of each segment were taken. By using this method percentage of free Na99mTcO4−, reduced/hydrolysed 99mTc, and the complex formed between 99mTc and Met-ac-TE3A/Trp-ac-TE3A conjugate could be calculated. Met-ac-TE3A/Trp-ac-TE3A conjugate remained at the origin, and free technetium traveled with the solvent front in acetone.
The radio conjugate was then purified using a C-18 reversed-phase extraction cartridge, which was preconditioned with 10 mL methanol and subsequently activated with 30% methanol to make prepare it more appropriate in terms of purity. The cartridge was successively rinsed with 5 mL distilled water, and radiolabeled conjugate was eluted in 5 mL of 5% ethanol.
2.5. In vitro studies
In tumor cell both the ligands were found intact even after 6 h and no other polar metabolite compound was found in hplc chromatograms.
2.6. In vivo studies
3. Result and discussion
3.1. Synthesis and radiochemistry of Met-ac-TE3A/Trp-ac-TE3A
In an attempt to develop target specific macrocyclic vehicle, two novel ligands Trp-ac-TETA, 6 and Met-ac-TE3A,7 were synthesized as Scheme 1. The cyclam was first converted into 1 by the reaction of tert-butylbromoacetate on 1,4,8,11-tetraazacyclotetradecane in 75% yield using a modified procedure reported in literature.16 The slow addition of tert-butylbromoacetate at 0 °C and purification by column chromatography led to appreciable increase in the final yield. Methyl-2-(2-chloroacetamido)-4-(methylthio) butanoate (2) was prepared by the reaction of methyl ester of methionine and chloroacetyl chloride in presence of base K2CO3. Similarly methyl-2-(2-chloroacetamido)-3-(1H-indol-3-yl) propionate (3) was also obtained as a colorless oil. These two intermediates were treated with 1 in presence of base to give tri-tert-butyl-2,2,2-(11-(3-((1-methoxy-4-(methylthio)-)-1-oxobutan-2yl)amino)-2-oxoethyl)-1,4,8,11-tetraaza-cyclotetradecane-(1,4,8-triyl)triacetate (4) and tri-tert-butyl-2,2,2-(11-((3-(1H-indol-3-yl)-1-methoxy-1-oxopropan-2yl)amino)-2-oxoethyl)-1,4,8,11-tetraaza-cyclotetradecane-(1,4,8-triyl)triacetate (5), which were further deprotected to get final product (6) and (7). 1H NMR spectra of 6 was showing α-H at 4.82–4.87 ppm and heterocyclic ring of tryptophan between 7.08 to 7.52 ppm while for 7 characteristic multiplet of amino acid backbone α-H at 4.58–4.63 ppm and S–CH3 at 2.11 ppm. In the 13C spectrum, the peak at 165 ppm represented the carbonyl carbon of COOH in both 6 and 7 and peak at 80 ppm tertiary carbon disappeared after deprotection of COOH group.The compounds were radiolabeled with 99mTc using SnCl2 as reducing agent. All the labeling parameters such as pH, concentration of reducing agent etc. were standardized, to achieve the maximum labeling efficiency. In vitro and in vivo stability of the labeled complexes was checked and the complexes were found to be stable for 24 h under physiological conditions. The labeling yield was found to be greater than 95%, as determined chromatographically by different solvent systems. The reaction mixture was kept in saline for different time intervals to carry out in vitro stability studies. Percentage radiolabeling was calculated for 0, 2, 4, 6, and 24 h. The metal binding assay confirmed the radiochemical purity to be >99%. Radiochromatogram showed a single peak for both the ligands which was found intact over 6 h.
3.2. pH-potentiometric studies
The metal complexes of macrocyclic ligands must be chemically stable under physiological conditions to ensure a desired biodistribution of the conjugates. This is also important to avoid the release of potentially toxic metal ions and transchelation of endogenous metal ions. Thus, such complexes should show high thermodynamic stability as well as strong kinetic inertness to dissociation, and the latter is generally favored in macrocyclic ligand. The prerequisite condition for synthesized ligands 6 and 7 to be used as Cu based PET radiopharmaceuticals and possible agents for lanthanide complexes in MR and other optical uses; it should form a highly stable complex with these metals in vitro. The values of the overall protonation constants and stability constants of their copper and europium complexes are presented in (Tables 1 and 2) respectively and compared with other known vehicles DTPA and DOTA.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β ± SD, n = 3) of the ligands DOTA and Trp-ac-TE3A and Met-ac-TE3A with the metal ions EuIII and ZnII and CuII (0.10 M KCl, at 25 °C)a
β ± SD, n = 3) of the ligands DOTA and Trp-ac-TE3A and Met-ac-TE3A with the metal ions EuIII and ZnII and CuII (0.10 M KCl, at 25 °C)a
| Stability constant | DOTA30b | Trp-ac-TE3A | Met-ac-TE3A |
|---|---|---|---|
| 0.1 M KCl | 0.1 M KCl | 0.1 M KCl | |
| a Calculated in separate reaction condition. | |||
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βEuL βEuL |
24.5 | 18.9 | 17.6 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βZnL βZnL |
18.1 | 16.8 | 15.3 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βCuL βCuL |
— | 20.4 | 19.2 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βCu2L βCu2L |
22.2 | 6.2 | 5.1 |
As anticipated, first and second protonation constants of the ligand were high, viz. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K1 and log
K1 and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K2 were 10.40 and 8.1 for Trp-ac-TE3A and 10.25 and 8.80 for Met-ac-TE3A. In general these two protonation constant are assigned to two trans group of macrocyclic backbone. Only mononuclear complexes could be found with copper, something that is understandable in view of the metal-to ligand ratio of 1
K2 were 10.40 and 8.1 for Trp-ac-TE3A and 10.25 and 8.80 for Met-ac-TE3A. In general these two protonation constant are assigned to two trans group of macrocyclic backbone. Only mononuclear complexes could be found with copper, something that is understandable in view of the metal-to ligand ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 that was used. The metal ligand ratios were 2
1 that was used. The metal ligand ratios were 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Titration for each ratio was carried out at least three times. It was likely that the presence of an excess of metal ions would lead to the formation of dinuclear complexes, but low stability constants of such species diminishes such possibility.
2. Titration for each ratio was carried out at least three times. It was likely that the presence of an excess of metal ions would lead to the formation of dinuclear complexes, but low stability constants of such species diminishes such possibility.
The values of the stability constants with copper was high for both the ligands and differs from those determined for Zn2+ and Eu3+ ions (Table 2). The values are comparable with those found for other similar ligands and are mainly determined by overall basicity of the nitrogen atoms (pK1 + pK2). The detail natures of the species are also represented in species distribution curve (ESI Fig. 3 and 4†).
3.3. In vitro studies
3.4. In vivo studies
 | ||
| Fig. 3 Whole-body γ image of balb/c mice with subcutaneous EAT tumor above the right hind leg at 4 h after i.v. injection of 99mTc [Met-ac-TE3A] and 99mTc [Trp-ac-TE3A]. | ||
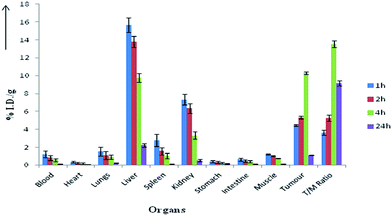 | ||
| Fig. 5 Tissue distribution data (% ID g−1 organ) of 99mTc [Met-ac-TE3A] in at 1 h, 2 h, 4 h and 24 h after i.v. administration in tumor bearing mice (n = 3). | ||
4. Conclusion
We have designed and developed possible biocompatible probes which can diagnose the tumor non-invasively and also provide a platform for the further development of multimodal imaging tools. Two novel amino acid based ligands Met-ac-TE3A and Trp-ac-TE3A were successfully synthesized and well characterized through spectroscopic techniques. In the preliminary studies they have shown good prospect as SPECT agent with 99mTc, which may extrapolated for 64Cu and 67Cu. The high stability of the compound with metal ions EuIII and CuII further expands its possible application from diagnosis to therapy as well as MR agent.Acknowledgements
We thank Dr R. P. Tripathi, Director INMAS, for providing necessary facilities. The work was supported by Defence Research and Development Organization, Ministry of Defence, under R&D project INM-311.References
- C. Huang and J. McConathy, J. Nucl. Med., 2013, 54, 1007–1010 CrossRef CAS PubMed.
- K. J. N. Isselbacher, Engl. J. Med., 1972, 286, 929–933 CrossRef CAS PubMed.
- K. J. N. Isselbacher, Proc. Natl. Acad. Sci. U. S. A., 1972, 69, 585–589 CrossRef CAS.
- T. Miyagawa, T. Oku and H. Uehara, J. Cereb. Blood Flow Metab., 1998, 18, 500–509 CrossRef CAS PubMed.
- D. Comar, J. C. Cartron, M. Maziere and C. Marazano, Eur. J. Nucl. Med., 1976, 1, 11–14 CrossRef CAS.
- B. Långstrom, G. Antoni and P. Gulberg, J. Nucl. Med., 1987, 28, 1037–1040 Search PubMed.
- K. Ishiwata, W. Vaalburg, P. H. Elsinga, A. M. J. Paans and M. G. Woldring, J. Nucl. Med., 1988, 29, 1419–1427 CAS.
- J. K. Chung, K. Kim, S. K. Kim, Y. J. Lee, S. Paek, J. S. Yeo, J. M. Jeong, D. S. Lee, H. W. Jung and M. C. Lee, Eur. J. Nucl. Med. Mol. Imaging, 2002, 29, 176–182 CrossRef CAS.
- T. Singhal, T. K. Narayanan, V. Jain, J. Mukherjee and J. Mantil, Mol. Imag. Biol., 2008, 10, 1–18 CrossRef PubMed.
- K. Higashi, A. C. Clavo and R. L. Wahl, J. Nucl. Med., 1993, 34, 773–779 CAS.
- D. H. Munn and A. L. Mellor, Trends Mol. Med., 2004, 10, 15–18 CrossRef CAS PubMed.
- C. Uyttenhove, L. Pilotte, I. Theate and J. Benoit, Nat. Med., 2003, 9, 1269–1274 CrossRef CAS PubMed.
- A. Mishra, J. Pfeuffer, R. Mishra, J. Engelmann, A. K. Mishra, K. Ugurbil and N. K. Logothetis, Bioconjugate Chem., 2006, 17, 773–780 CrossRef CAS PubMed.
- P. P. Hazari, G. Shukla, V. Goel, K. Chuttani, N. Kumar, R. Sharma and A. K. Mishra, Bioconjugate Chem., 2010, 21, 229–239 CrossRef CAS PubMed.
- K. G. Kadiyala, A. Datta, J. Tanwar, A. Adhikari, B. S. Kumar, K. Chuttani, M. Thirumal and A. K. Mishra, Pharm. Res., 2015, 32(3), 955–967 CrossRef CAS PubMed.
- J. K. Uppal, P. P. Hazari, C. K. Raunak, M. Allard, N. K. Kaushik and A. K. Mishra, Org. Biomol. Chem., 2011, 9, 1591–1599 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra07138k |
| This journal is © The Royal Society of Chemistry 2015 |





