Natural-photosynthesis-inspired photovoltaic cells using carotenoid aggregates as electron donors and chlorophyll derivatives as electron acceptors†
Abstract
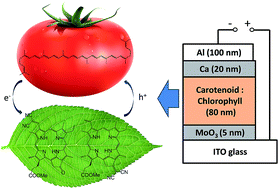
Carotenoids (Cars) and chlorophylls (Chls) are major pigments playing key roles in light-harvesting and energy transfer processes in natural photosynthetic apparatus. We demonstrated, in this study, photovoltaic cells with entire active layers consisting of a linear Car, lycopene, as the electron donor and Chl derivatives, either methyl 32,32-dicyano-pyropheophorbide-a (Chl-1) or methyl 131-deoxo-131-(dicyanomethylene)pyropheophorbide-a (Chl-2), as the electron acceptor.

 Please wait while we load your content...
Please wait while we load your content...