A eutectic mixture of choline chloride and urea as an assisting solvent in the synthesis of flower-like hierarchical BiOCl structures with enhanced photocatalytic activity
Abstract
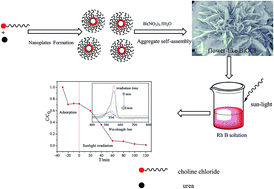
Flower-like hierarchical BiOCl structures were successfully synthesized in the presence of a eutectic mixture of choline chloride and urea through a solvothermal process. The as-prepared sample was characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), X-ray photoelectron spectroscopy (XPS), N2 adsorption–desorption analysis, photo-electrochemical analysis and UV-vis diffuse reflectance spectroscopy (DRS). During the reaction process, the eutectic mixture of choline chloride and urea acted not only as the solvent and the template, but also as a chlorine source for the fabrication of flower-like BiOCl. In addition, the photocatalytic activity toward the degradation of RhB in aqueous solution was also investigated. The results showed that the flower-like BiOCl exhibited enhanced photocatalytic activity for the degradation of RhB under sunlight illumination. Meanwhile, the flower-like BiOCl catalyst performed without obvious decrease in activity after five cycles. Furthermore, the main reactive species in the catalytic oxidation reaction were detected through radical trapping experiments.

 Please wait while we load your content...
Please wait while we load your content...