The mechanism of 2,4,6-trinitrotoluene detection with amino acid-capped quantum dots: a density functional theory study†
Abstract
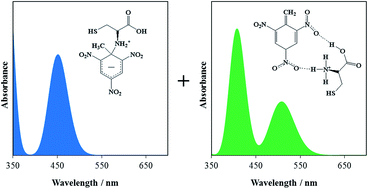
When an amino acid-capped quantum dots solution meets 2,4,6-trinitrotoluene (TNT), it changes from colorless to red and its fluorescence is quenched. This is a recently developed technique for the detection of TNT in trace amounts. However, what causes the changes in coloration and fluorescence remains controversial. Using density functional theory calculations, we studied the structures and optical properties of the products of TNT reacting with cysteine. Two compounds, namely, a Meisenheimer complex and a TNT anion, which are obtained from an addition reaction and an acid–base reaction respectively, were characterized, but neither of them could be used solely to interpret the experimental results. Our calculations proposed the possibility of their coexistence in the solution from their similar thermodynamic stability and their predicted absorption and vibrational spectra. The superposition of their calculated optical absorption spectra produces band distributions similar to those of the experiments. Moreover, the measured Raman spectra that had been used to characterize the formation of the Meisenheimer complex cannot exclude the formation of the TNT anion whose characteristic vibrations are buried by those of the former. Our calculations also revealed that in the Meisenheimer complex the electron delocalization in the phenyl ring of TNT is blocked by the attached cysteine, while for the TNT anion, the removal of the hydrogen atom enhances the electron delocalization and leads to a redshift of its first excitation in comparison with that of the Meisenheimer complex. Therefore, the key to TNT detection is to control the size of the QDs so as to adjust their emission to a wavelength around the absorption bands of either the Meisenheimer complex or the TNT anion that are formed with TNT and the amino acid.

 Please wait while we load your content...
Please wait while we load your content...