Novel nanocomposite hydrogel for wound dressing and other medical applications
Ali Hebeish and
S. Sharaf*
National Research Centre, Textile Division, Textile Chemistry and Technology, Department of Preparation and Finishing of Cellulosic Fibers, 33 El Bohouth St.-Dokki-Giza, Cairo, P.O. Box 12622, Giza, Egypt. E-mail: samarsami2004@yahoo.com; Tel: +20 1001967044
First published on 30th October 2015
Abstract
Graft copolymerization of diallyldimethylammonium chloride (DADMAC) vinyl monomer together with N,N′-methylene-bis-acrylamide (MBA) crosslinking agent onto water soluble carboxymethyl cellulose (CMC) was carried out using ammonium persulfate (APS) initiator. The copolymerization resulted in the formation of hydrogels. The characteristics and properties of these hydrogels were dependent on the conditions affecting the copolymerization reaction and these, in turn, controlled the pore size and porous structure of the hydrogels. Thus, increasing the monomer concentration caused a major enhancement in the swelling ratio of the hydrogel provided that the monomer was used at a concentration of 40% or more. The opposite was true for initiator concentration: the swelling ratio of the hydrogel decreased significantly by increasing APS concentration from 0.05 to 0.25 mol L−1. With respect to MBA crosslinker, a maximum swelling ratio of 30 could be achieved with hydrogel prepared using MBA at a concentration of 0.1 mol L−1; hydrogel prepared in the presence of MBA at 0.05 mol L−1 exhibited zero swelling ratio while hydrogel prepared using MBA at 0.3 mol L−1 displayed a swelling ratio of 10%. The maximum swelling ratio for hydrogel was achieved at pH 7 and a significant decrease in the swelling ratio of hydrogel was observed within the pH range 2–6 as well as at pH 8. The hydrogel could also be successfully attached to modified cotton fabric, namely partially carboxymethylated cotton (PCMC) through ionic crosslinking. The in situ formation of CuO nanoparticles inside the matrix of CMC–DADMAC nanocomposite hydrogel attached to cotton fabric was also investigated and was confirmed using X-ray diffraction and scanning electron microscopy studies. Furthermore, the functional performance of the novel CuO nanocomposite hydrogel as wound dressing was tested for antibacterial activities; the nanocomposite hydrogels demonstrated excellent antibacterial effect. The work was further extended to include the synthesis and characterization of Ag/CMC–DADMAC nanocomposite hydrogel. The latter displayed high antibacterial activity.
1. Introduction
Hydrogels, also known as super absorbents, are preferably synthesized by grafting vinyl monomers onto natural polysaccharides and then compounding with inorganic nanoscale metals. This approach forms the basis of the method of choice because it affords unique environmental and commercial advantages. Up to now, most dual temperature and pH-sensitive hydrogels that can swell in an acidic pH environment and deswell at alkaline pH have found applications in cases such as drug release and dye adsorption.1–4 For example, the drug (chloramphenicol) must be released more rapidly from hydrogel in a pH 1.4 buffered solution (close to the pH of the stomach) than in a pH 7.4 environment (close to the pH of the intestine),1 in which the drug release is controlled by the swelling/deswelling behavior of the hydrogel. To achieve such functions, cationic hydrogels are needed. Diallyldimethylammonium chloride (DADMAC) is a water-soluble quaternary ammonium compound that can be cyclopolymerized to its corresponding polymer and is widely used in water treatment, paper manufacturing, mining, and biology.5 It was found in previous studies that polyDADMAC hydrogel could absorb water several hundred times its dry weight.6,7 Furthermore, the quaternary ammonium compound is antibacterial which is advantageous in medical applications.8Among all cellulose ethers, only carboxymethyl cellulose (CMC), available as the sodium salt (NaCMC), is a polyelectrolyte, and thus a smart cellulose derivative which shows sensitivity to pH and ionic-strength variations, plus good swelling capability.7,9
As textiles become more functional, stimuli-responsive polymers have also found their application in the creation of intelligent or smart textiles. These environmentally responsive fabrics can be tailored by chemical modification of the textile's surface using polymeric chains. Smart textiles may provide us with considerable convenience, support, and even pleasure, in our daily activities.10
Hydrogel-based hybrid materials incorporating an inorganic phase in the form of nanoparticles (NPs) are receiving an increasing amount of attention, thanks to the synergic properties of the hydrogels and their inorganic components.11 Different types of inorganic nanoparticles have been incorporated to prepare hydrogel-based hybrid systems with tailored mechanical or functional properties.12–14 Hydrogels can afford free-space between the networks in the swollen stage that serve for nucleation and growth of nanoparticles and act as nanoreactors or nanopots. This approach was established by Wang et al.15 and Murali Mohan et al.16 to obtain 3–5 nm sized gold and silver nanoparticles within the poly(N-isopropylacrylamide) (PNIPAM) based hydrogel networks. Much research and development effort has been devoted to the production of hydrogels containing metal nanoparticles which are highly suitable for biomedical applications.17
Very recently, the development of different stimuli-responsive hydrogels was the subject of our research activities. For example, thermal responsive hydrogels based on a semi-interpenetrating network of poly(N-isopropylacrylamide) (poly(NIPAM)) and cellulose nanowhiskers were discussed.18 Investigations into the synthesis and characterization of novel CMC hydrogels and CMC hydrogel–ZnO-nanocomposites were performed.19 We have also reported on the development of CMC hydrogels loaded with silver nanoparticles for medical applications.17
The current research has been undertaken with a view to developing smart textiles with tunable water absorbance, changing with the environment. The development of such a textile hydrogel is based on radical solution polymerization of DADMAC monomer on to carboxymethylcellulose (CMC) using ammonium persulfate (APS) as an initiator and N,N′-methylene-bis-acrylamide (MBA) as a crosslinking agent. To the best of our knowledge, this copolymer has not been used before in textile applications or as a carrier for nanoparticles. Copolymerization to achieve hydrogel formation was carried out under a variety of conditions as was the application of the hydrogels to partially carboxymethylated cotton (PCMC) fabric. State of the art facilities were used for analysis and characterization of the products obtained. We prepared a novel wound dressing containing CuO/CMC–DADMAC nanocomposite hydrogel as well as Ag/CMC–DADMAC nanocomposite hydrogel.
2. Experimental
2.1 Materials
Mill-scoured and bleached cotton fabric was kindly supplied by Misr Co. for spinning and weaving, Mehala El kubra, Egypt.Carboxymethyl cellulose (CMC) (Mw = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da), diallyldimethylammonium chloride (DADMAC; 97%) (Merck, Germany), N,N′-methylene-bis-acrylamide (MBA; 99%), ammonium persulfate (APS; 98%), and all other chemicals were of laboratory grade.
000 Da), diallyldimethylammonium chloride (DADMAC; 97%) (Merck, Germany), N,N′-methylene-bis-acrylamide (MBA; 99%), ammonium persulfate (APS; 98%), and all other chemicals were of laboratory grade.
2.2 Method
2.3 Characterization and analysis
| Q = We/Wd |
3. Results and discussion
3.1 Mechanism of hydrogel formation
Initially, the ammonium persulfate (APS) initiator is decomposed under heating to generate sulfate anion radicals. The radicals extract hydrogen from the hydroxyl group of the sodium carboxymethylcellulose to form alkoxy radicals on the substrate. The monomer molecules, which are in close proximity to the reaction sites, become acceptors of carboxymethylcellulose radicals resulting in chain initiation and thereafter themselves become free radical donors to neighboring molecules. In this way, the grafted chain grows.22,23 Since a crosslinking agent, i.e. MBA, is present in the system, the end vinyl groups of the MBA crosslinker may react synchronously with polymer chains during chain propagation. The copolymer consists of a crosslinked structure. DADMAC forms mostly via five-member ring formation. Scheme 1 shows the two structures, i.e. structure 1 and structure 2, suggested for DADMAC monomer. Also shown is the mechanism of formation for CMC–poly(DADMAC) crosslinked copolymer hydrogel along with the reactions involved therein. | ||
| Scheme 1 Mechanism for CMC–poly(DADMAC) hydrogel synthesis where structures 1 and 2 represent the proposed structures for DADMAC monomer. | ||
3.2 Hydrogel characterization
3.3 Synthesis of CMC/DADMAC hydrogels: effect of process parameters
In neutral water as the swelling medium, CMC is a negatively charged polyelectrolyte in the swelling system, and the strong electrostatic repulsions among CMC carboxylate anions (COO−) could result in a more expanded network in the hydrogel. The latter assumes the highest swelling ratio at pH 7, a point which could be associated with an increasing number of ionic groups in the hydrogels which causes an increment in their swelling capacity due to additional osmotic pressure provided by counterions inside the gel. However, the swollen gel rapidly shrinks because of protonation of –COO− groups under acidic pH values (pH < 5), where most of the carboxylate anions are protonated. On the one hand, the hydrogen-bonding interaction among carboxylate groups is strengthened and additional physical crosslinking is generated. As a result, the network tends to shrink and consequently swelling values are decreased. The decreased absorbency at higher basic pH values (pH > 8) is related to the ‘screening effect’ of excess cations in the swelling media.
3.4 Functionalization of cotton textile by CMC–DADMAC copolymer hydrogels
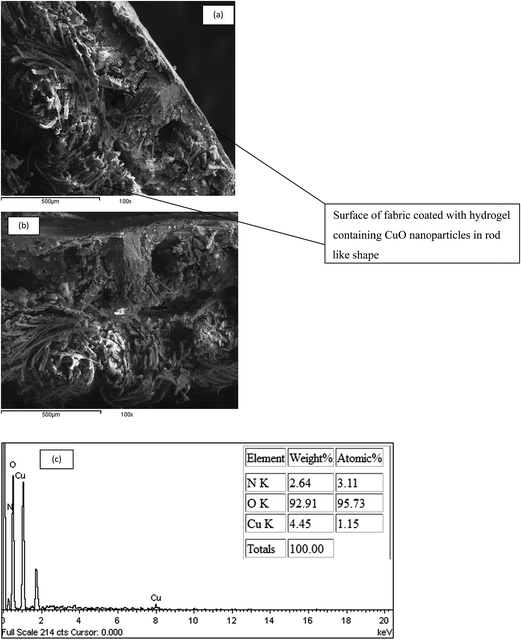
The main challenge in developing smart textile materials is confined to techniques for successful attachment of the hydrogel layer to the textile substrate. Recent research has disclosed that hydrogel particles can be covalently bonded to cotton using appropriate crosslinking agents.25 In the current work, the hydrogel was attached to the surface of partially carboxymethylated cotton (PCMC) fabric through ionic crosslinking. Anionic PCMC fabric was synthesized by reacting it with monochloroacetic acid in alkaline medium. This method not only keeps the elastic form of the hydrogel but also confirms its attachment to the cotton fabric. The presence of hydrogel on the surface of PCMC fabric is indeed visually confirmed by SEM.The surface morphology of PCMC fabric coated with the hydrogel is shown in Fig. 9. As can be seen from Fig. 9a–c, the fibers are covered with irregular fragments, rendering the surface of the fabric rough and homogeneous. This means a thin hydrogel layer is coating the fabric. Fig. 9d illustrates the surface morphology (cross-section) of the fabric coated with a thin layer of hydrogel.
By studying the swelling characteristics of cotton–hydrogel samples, we have concluded that the incorporation of hydrogel onto the surface of the cotton fabric causes significant changes in its swelling behavior. Most probably, the ionic crosslinking between PCMC and the cationic hydrogel decreases the affinity of the hydrogel towards water and the swelling ratio decreases to a value of 10 at pH 7, while in both acidic (pH 5) and alkaline (pH 9) environments, the swelling ratio decreases to a value of 8. This decrease at particular pH values can be attributed to the action of the concentrated solution of caustic soda during functionalisation of the cotton surface. However, the hydrogel-coated cotton still acquires good swelling characteristics.
3.5 Preparation of carboxymethylcellulose/CuO bio-nanocomposite hydrogels
CMC interacts with many metal cations including Al3+, Cu2+, Co2+, Mo6+, and Zn2+,26 due to the porous structure of hydrogels and the existence of carboxylate groups (–CO2−), thus the CMC hydrogels can easily bind to the Cu2+ cations in aqueous solutions of copper sulphate via electrostatic interactions. With a suitable basic agent such as NaOH, copper ions are oxidized to CuO nanoparticles. The reaction process can be expressed as follows:| Cu2+ + 4HN3·H2O → [Cu(NH3)4]2+ + 4H2O | (1) |
| [Cu(NH3)4]2+ + 2NaOH → Cu(OH)2↓ + 4HN3 + 2Na2+ | (2) |
| Cu(OH)2 → CuO + H2O | (3) |
3.5.1.1 X-ray diffraction (XRD) analysis. The X-ray diffractogram of CuO/CMC nanocomposite hydrogel on fabric in the 2θ range of 2–70° is shown in Fig. 10. The diffractogram of CMC/CuO nanocomposite hydrogel is characterized by diffractions at 2θ values at about 35°, 38°, 49°, 53°, 58°, and 62° which are assigned to the (110), (002), (112), (020), (202) and (−311) diffractions of CuO crystals, respectively. All of the peaks match well with those of monoclinic phase CuO crystals and confirm the formation of CuO particles in the CMC hydrogel matrix.
3.5.1.2 Scanning electron microscopy (SEM). In comparison with Fig. 9d which showed the surface morphology of cotton coated with a hydrogel layer, Fig. 11a,b shows SEM images of cotton fabric coated with CMC hydrogel containing CuO nanoparticles. It was observed that a large amount of needle-like aggregates of CuO nanoparticles are trapped within the hydrogel matrix forming a homogenous layer on the surface of the cotton fabric and at depth inside it.
3.5.1.3 EDS analysis. The EDS spectrum of CuO nanoparticles (shown in Fig. 11c) clearly demonstrates the presence of Cu and O peaks with 4.45 wt% and 92.9 wt%, respectively, confirming the presence of CuO nanoparticles, which is consistent with the XRD results.
3.6 Synthesis and characterization of silver nanocomposite hydrogel (Ag/CMC–DADMAC)
When a fully swollen CMC–DADMAC hydrogel in the form of a disk is put in aqueous AgNO3 solution, Ag+ ions replace the H+ or Na+ ions in the CMC hydrogel. Therefore, Ag+ ions are still accessible for reduction into nanosilver by sodium borohydride solution forming silver nanoparticles within the swollen network as illustrated in Fig. 12. | ||
| Fig. 12 Schematic representation of steps involved in preparation of Ag/CMC–DADMAC nanocomposite hydrogel. | ||
Fig. 13b and c also supports the view that the silver nanoparticles are formed throughout the network and along with the polymeric network in addition to existing in free spaces in the networks. This means that the hydrogel acts as a reactor for silver nanoparticles that grow as bright dots distributed between the gel networks with the help of the polymeric chains. This can be seen where the silver nanoparticles are overlying on CMC–DADMAC copolymer chains in the hydrogel network. The Energy Dispersive X-ray Spectroscopy (EDX) of the freeze-dried sample of the prepared hydrogel containing silver nanoparticles (Fig. 13c and d) shows that the Ag nanoparticles are loaded inside the matrix of the hydrogel with high content (71 wt% Ag) with a uniform spatial distribution of Ag nanoparticles on CMC–DADMAC nanocomposite hydrogel.
3.7 Antibacterial activity
The antibacterial activities of CuO/CMC–DADMAC dressing, Ag/CMC–DADMAC nanocomposite hydrogel, and CMC–DADMAC (control sample) were studied against Gram-positive and Gram-negative bacteria. The antibacterial activity was determined in terms of the size of the inhibition zones on agar medium. It was observed that the control sample did not display any antibacterial activity despite the presence of the quaternary ammonium groups in the copolymer hydrogel.27–29 The effectiveness of the quaternary ammonium groups as an antibacterial seems to be abolished through their intimate association and interaction with CMC in the copolymer hydrogel. That is why the CMC–DADMAC copolymer hydrogel failed to induce antibacterial activity. On the other hand, CuO/CMC–DADMAC nanocomposite hydrogel and Ag/CMC–DADMAC nanocomposite hydrogel can release copper and silver nanoparticles into the pathogenic environment,30 thereby producing highly efficient antibacterial activity (Table 1).| Sample | Inhibition zone diameter (mm cm−1) sample | |||
|---|---|---|---|---|
| E. coli (G –ve) | P. aeruginosa (G –ve) | S. aureus (G +ve) | B. subtilis (G +ve) | |
| a All experimental test data were collected in triplicate, and the average value taken. | ||||
| CMC–DADMAC dressing hydrogel | Zero | Zero | Zero | Zero |
| CuO/CMC–DADMAC dressing hydrogel | 19 | 20 | 20 | 18 |
| Ag/CMC–DADMAC hydrogel | 17 | 19 | 18 | 17 |
The results of Table 1 show decisively that both CuO/CMC–DADMAC and Ag/CMC–DADMAC nanocomposite hydrogels acquire high antibacterial activity. The inhibition zones exhibit values of 19 and 20 mm cm−1 for CuO nanocomposite hydrogel dressing and 17 and 19 mm cm−1 for Ag nanocomposite hydrogels upon using E. coli (G –ve) and P. aeruginosa (G –ve) bacteria, respectively. When using S. aureus (G +ve) and B. subtilis (G +ve) bacteria, the values were, respectively, 20 and 18 mm cm−1 for CuO nanocomposite hydrogel dressing and 18 and 17 mm cm−1 in the case of Ag nanocomposite hydrogel.
Within this range of studies, it is logical to assume that the antibacterial activity of the materials under investigation relies, in essence, on the nature of the nanoparticles under investigation as well as on the bacteria which, in turn, determine the speed and mechanism of release of copper oxide or silver ions from the nanoparticles of the nanocomposite hydrogel and the interaction of the released ions with the cell wall of the bacteria. On the other hand, in the case of CuO nanoparticles, the activity could be explained in terms of attachment of CuO nanoparticles to the cell wall of bacteria which damages the cell wall and causes leakage of proteins and other intracellular constituents and ultimately leads to cell death.30–32
In the case of the presence of Ag nanoparticles in the hydrogel matrix, it was reported that Ag nanoparticles penetrate the cell wall of Gram –ve bacteria.33,34 As a result, a structural change in the cell membrane occurs. This could lead to an increase in cell permeability which, in turn, leads to uncontrolled transport through the cytoplasm membrane and ultimately to the death of the cell. Another mechanism is based on free radical formation followed by free radical-induced damage to the cell membrane. It is also likely that silver ions move into the cell and, as a result, production of reactive oxygen species takes place which can damage the cell wall. It is further reported that there is a greater tendency for silver ions to interact with thiol groups of vital enzymes as well as phosphorous containing bases35 and, with the presence of silver nanoparticles inside the cells,36 it is logical that certain damage could be realized through interactions with compounds such as DNA. This interaction may stop cell division and DNA replication and end with death of the cell.
4. Conclusion
Hydrogels with unique properties were synthesized through copolymerization of CMC with DADMAC in the presence of APS initiator and MBA crosslinker. The pore size and porous structure of the hydrogels thus obtained could be controlled by making use of variables affecting the formation of the hydrogels. Briefly, CMC-DADMAC copolymer hydrogels had a higher swelling ratio when higher DADMAC monomer concentrations were used. The opposite held true for either APS or MBA where the hydrogels displayed lower swelling ratios with increasing concentrations of APS or MBA. Particularly notable is the plot of the results of swelling ratio versus pH. The swelling ratio of the hydrogel exhibited a striking decrease within the pH range 2–6 as well as at pH 8. On the other hand, a hydrogel with maximum swelling ratio could be achieved at pH 7. The hydrogels under investigation also form the basis for production of wound dressings. The hydrogel was attached to PCMC fabric via ionic crosslinking. Furthermore, the antimicrobial activity of the novel hydrogel was examined on Gram-negative and Gram-positive bacteria according to the agar diffusion test. The CuO/CMC–DADMAC nanocomposite hydrogels showed higher antibacterial activity than Ag/CMC–DADMAC nanocomposite hydrogels against Gram-positive and Gram-negative bacteria. Based on these findings, the prepared nanocomposite hydrogels can be used in different medical fields, i.e. drug delivery, wound dressing as well as wound healing.Acknowledgements
This project was supported financially by the Science and Technology Development Fund (STDF), Egypt, grant number 4384.References
- J. T. Guo, L. Li, X. Y. Li and J. L. Zhu, J. Appl. Polym. Sci., 2006, 100, 3602–3608 CrossRef CAS
.
- Y. L. Zhang, L. Xu, M. Yi, M. L. Zhai, J. R. Wang and H. F. Ha, Eur. Polym. J., 2006, 42, 2959–2967 CrossRef CAS
.
- D. Şoipan, M. Şen, Z. Klöge and O. Güven, Radiat. Phys. Chem., 2008, 77, 428–433 CrossRef
.
- J. Zhang, R. Xie, S. Zhang, C. Cheng, X.-J. Ju and L.-Y. Chu, Polymer, 2009, 50, 2516–2525 CrossRef CAS
.
- C. Wandrey and D. Hunkeler, Adv. Polym. Sci., 1999, 145, 123–182 CrossRef CAS
.
- N. Liu, M. Yi, S. J. Chen and H. Ha, Chin. J. Polym. Sci., 2002, 20(5), 409–412 CAS
.
- J. Ren and H. F. Ha, Eur. Polym. J., 2001, 2413–2417 CAS
.
- Y. Zhang, L. Xu, M. Yi, M. Zhai, J. Wang and H. Ha, Eur. Polym. J., 2006, 42, 2959–2967 CrossRef CAS
.
- A. Sannino, C. Demitri and M. Madaghiele, Design and applications, Materials, 2009, 2, 353–373 CrossRef CAS
.
- S. Gorgieva and V. Kokol, Carbohydr. Polym., 2011, 85, 664–673 CrossRef CAS
.
- L. Zhang and T. Webster, Nano Today, 2009, 4, 66–80 CrossRef CAS
.
- Y. Murali Mohan, K. Vimala, V. Thomas, K. Varaprasad, B. Sridhar and S. K. Bajpai, et al, J. Colloid Interface Sci., 2010, 342, 73–82 CrossRef CAS PubMed
.
- R. Bilgainya, F. Khan and S. Mann, Mater Sci Eng C, 2010, 30, 352–356 CrossRef CAS
.
- H. P. Cong and S. H. Yu, Curr. Opin. Colloid Interface Sci., 2009, 14, 71–80 CrossRef CAS
.
- C. Wang, N. T. Flynn and R. Langer, Adv. Mater, 2004, 16, 1074 CrossRef CAS
.
- Y. Murali Mohan, T. Premkumar, K. Lee and K. E. Geckeler, Macromol. Rapid Commun., 2006, 27, 1346 CrossRef
.
- A. Hebeish, M. Hashem, M. M. Abd El-Hady and S. Sharaf, Carbohydr. Polym., 2013, 92, 407–413 CrossRef CAS PubMed
.
- A. Hebeish, S. Farag, S. Sharaf and Th. I. Shaheen, Carbohydr. Polym., 2014, 102, 159–166 CrossRef CAS PubMed
.
- M. Hashem, S. Sharaf, M. M. Abd El-Hady and A. Hebeish, Carbohydr. Polym., 2013, 95, 421–427 CrossRef CAS PubMed
.
- M. Hashem, R. Refaie and A. Hebeish, Journal of Cleaner Production, 2005, 13, 947e954 CrossRef
.
- A. Pourjavadi, M. J. Zohuriaan-Mehr, S. N. Ghasempoori and H. Hossienzadeh, J. Appl. Polym. Sci., 2007, 103, 877–883 CrossRef CAS
.
- A. Pourjavadi, M. Ayyari and M. S. Amini-Fazl, Eur. Polym. J., 2008, 44(4), 1209–1216 CrossRef CAS
.
- S. Korpe, B. Erdoğ, G. Bayram, S. Ozgen, Y. Uludag and N. Bicak, React. Funct. Polym., 2009, 69, 660–665 CrossRef CAS
.
- P. J. Flory, Principles of Polymer Chemistry, Cornell University, Ithaca, NY, 1953 Search PubMed
.
- A. Basharia, N. Hemmatinejada and A. Pourjavadib, Polym. Adv. Technol., 2013, 24, 797–806 CrossRef
.
- A. P. Franco, M. A. L. Recio, B. Szpoganicz, A. L. Delgado, J. Felcman and A. L. R. Mercê, Hydrometallurgy, 2007, 87, 178–189 CrossRef CAS
.
- W. J. Ye, M. F. Leung, J. Xin, T. L. Kwong, D. K. L. Lee and P. Li, Polymer, 2005, 46, 10538 CrossRef CAS
.
- S. Lenoir, C. Pagnoulle, C. Detrembleur, M. Galleni and R. Jerome, J. Polym. Sci.Polym. Chem., 2006, 44, 1214 CrossRef CAS
.
- C. Z. S. Chen, N. C. Beck-Tan, P. Dhurjati, T. K. Dyk, R. A. LaRossa and S. L. Cooper, Biomacromolecules, 2000, 1(3), 473–480 CrossRef CAS PubMed
.
- B. Reidy, A. Haase, A. Luch, K. A. Dawson and I. Lynch, Materials, 2013, 6, 2295–2350, DOI:10.3390/ma6062295
.
- A. P. Ingle, N. Duran and M. Rai, Appl. Microbial. Biot, 2013, 98, 1–9 Search PubMed
.
- D. Das, B. C. Nath, P. Phukon and S. K. Dolui, Colloid. Surf. B, 2013, 101, 430–433 CrossRef CAS PubMed
.
- K. Gopalakrishnan, C. Ramesh, V. Ragunathan and M. Thamilselvan, Dig. J. Nano-mater. Biol., 2012, 7(2), 833–839 Search PubMed
.
- J. R. Morones, J. L. Elechiguerra, A. Camacho, K. Holt, J. B. Kouri and J. T. Ramirez, et al., Nanotechnology., 2005, 16(10), 2346–2353 CrossRef CAS PubMed
.
- I. Sondi and B. Salopek-Sondi, J. Colloid Interface Sci., 2004, 275(1), 177–182 CrossRef CAS PubMed
.
- W. Hatchett and S. White, J. Phys. Chem., 1996, 100(23), 9854–9859 CrossRef
.
| This journal is © The Royal Society of Chemistry 2015 |