Spectral properties and luminescence mechanism of red emitting BCNO phosphors†
Xinghua Zhang*ab,
Xiaobo Jiaab,
Hui Liuab,
Zunming Luab,
Xiaokai Maab,
Fanbin Mengab,
Jianling Zhaoab and
Chengchun Tangab
aSchool of Materials Science and Engineering, Hebei University of Technology, Tianjin, China. E-mail: xinghua146@aliyun.com; Fax: +86-022-60202660; Tel: +86-022-60204805
bKey Laboratory of Micro- and Nano-scale Boron Nitride Materials of Hebei Province, China
First published on 29th April 2015
Abstract
Red emitting (λem = 620 nm) BCNO phosphors were synthesized at 650 °C with solid state reaction method using boric acid and hexamethy lenetetramine as raw materials. The BCNO phosphors have turbostratic boron nitride structure and particle sizes are in micro scale. Carbon and oxygen elements were bonded to boron and nitrogen to form BCNO phosphors. The emission peaks were shifted from blue light (420–470 nm) to red light (590–620 nm) with increasing sintering temperature, heating time and the ratio of boric acid to hexamethy lenetetramine, which was induced by partially formed BCNO and completely formed BCNO phosphors. The decay curves and emission spectra indicated that the red emission was induced by two luminescence centers, corresponding to longer lifetime τ1 and short lifetime τ2. The ultraviolet visible absorption spectra disclosed that the optical band gap was changed from 1.75 eV to 2.0 eV with different preparation conditions. The high temperature emission spectra suggested that the nitrogen defects levels served as electron traps and attended the red emission. The luminescence mechanism of BCNO phosphors was stated by a simplified energy level diagram. The red emission BCNO phosphors have good thermal stability and great potential application on lighting, display, solar cell and biomedical fields.
Introduction
Red emitting phosphors have been paid much attention due to their ability to improve the color rendering index (CRI) and produce warm white light for white light emitting diodes (LEDs). Up to now, different red emitting phosphors have been developed such as Eu doped nitrogen compound (Sr2Si5N8:Eu2+)1a and sulfides (CaS:Eu2+),1b Mn doped compounds (CaMg2Al16O4:Mn4+)1c and some quantum dot materials (CdSe/CdS).1d It is still a challenge to develop new red emitting phosphors with pollution free, economize and energy saving. Hexagonal born nitride (BN) have been attracted much attention since the first observation of an intense far ultraviolet (FUV) excitation emission.2 Owing to the wide band gap of BN (∼5.9 eV), its luminescence was limited in short wavelength (∼215 nm). A great deal of work has been done for broadening the emission spectra of BN in visible light.3 A recent development of boron nitride based phosphors was performed by T. Ogi with preparing carbon and oxygen codoped boron nitride (BCNO) phosphors via urea combustion method.4a The BCNO phosphors can be synthesized from inexpensive and environmentally friendly raw materials with wide range of excitation from short ultraviolet to blue light (250–430 nm) and emission spectra from violet to near-red regions (387–571 nm), and high quantum yields.BCNO phosphors have been focused on owing to its great potential application in white light emitting diodes (LEDs), display, biology and medical imaging areas.4b At present, the preparation, spectral properties and luminescence mechanism of BCNO phosphors have been investigated preliminarily. For preparation, different methods were adopted to synthesize BCNO phosphors with various morphologies. Singh et al.5a prepared BCNO nanophosphor by solid state reaction method at 1400 °C with boric acid and melamine (λem = 357 nm). Lei et al.5b synthesized BCNO nanoparticles in a eutectic LiCl/KCl salt melt using sodium borohydride and urea as raw materials (λem = 440–528 nm). Our group used chemical method5c–e to prepare BCNO phosphors in micrometer size. In addition, BCNO nanofibers and porous microbelts were also synthesized by electrospinning5f and one-step template-free method, respectively.5g For spectral properties, Kaihatsu et al.6a investigated the effects of carbon source, sintering time and temperature, ratio of raw materials on luminescence spectra of BCNO phosphors. The emission spectra were modulated from 387 nm to 570 nm with increasing ratio of carbon source to boron source (0–1.02), and the emission spectra were shifted from 420 nm to 550 nm with decreasing ratio of nitrogen source to boron source (9.96–1.0). Ogi et al.6b disclosed the effects of polymer (polyethyleneimine, polyallylamine, tetraethylene glycol) on the quantum yields and photoluminescence properties of BCNO phosphors, and the emission of BCNO can be tuned from 380 to 490 nm by changing the reaction and polymer concentration. Lu et al.6c studied the influence of annealing temperature and ambient atmosphere on the photoluminescence properties of BCNO phosphors. Chu et al.6d tuned emission spectra of BCNO phosphor from blue to yellow by doping grapheme oxide into BCNO phosphors. For luminescence mechanism, there are mainly two points of view to clarify the luminescence origin of BCNO phosphors. One argument is that the luminescence of BCNO phosphor was induced by the closed shell BO2− and BO− species.7a,b Another suggestion7c is defects energy level transition which led to the luminescence of BCNO phosphors. At present, the mainly method to modulate the emission spectra was selected proper carbon source and increasing content of carbon source. However, it is difficult to modulate the emission peak to red light because the emission peak will shift back to short wavelength with too much carbon source. In addition, it was often adopted three sources (boron, nitrogen, carbon sources) to synthesize BCNO phosphor. The selection of carbon source is important and it is hard to control the carbon source during the preparation of BCNO phosphor. Up to now, there is no report to synthesize red emission BCNO phosphors with two sources, much less to investigate the spectral properties and luminescence mechanism of red emission BCNO phosphors. In addition, it is easier to control the reaction process and modulating spectral properties by changing the two sources, and solid state reaction method is favourable for realizing quantity production and industrialization.
In this paper, we prepared red emitting BCNO phosphors with solid state reaction method using boric acid and hexamethy lenetetramine as raw materials. The chemical bond status was investigated by X-ray photoelectrical spectra (XPS) and infrared spectra. The excitation spectra, emission spectra, and decay process were systematically measured to investigate the luminescence properties of BCNO phosphors. The optical band gap were studied by ultraviolet visible (UV-vis) absorption spectra and the high temperature emission spectra were used to investigate the thermal stability of BCNO phosphors and the electron transition process under heating situation. In addition, the luminescence mechanism of red emitting BCNO phosphors was also investigated.
Experimental procedures
The BCNO phosphors were prepared by a solid state reaction method using boric acid and hexamethy lenetetramine as raw materials. The proper carbon, nitrogen concentration and the inherent C–N bonds with heterocyclic structure was favourable for hexamethy lenetetramine using as a good choice of carbon and nitrogen source. Firstly, the boric acid (H3BO3, B source) and hexamethy lenetetramine (C6H12N4, C and N source) were weighed and ground sufficiently in an agate mortar. Then, the ground raw materials were pressed into a disc with 10 mm in diameter (2 mm thick) and sintered at 600–700 °C for 4–24 h in a ceramic crucible under ambient atmospheric pressure. The air condition was helpful for synthesizing BCNO phosphors and modulating the carbon and oxygen concentration in BCNO phosphors. Three series of BCNO samples were prepared with various sintering temperatures, heating times and different mole ratios of boron acid and hexamethy lenetetramine. For the first series of BCNO phosphor, the mole ratio of boron acid to hexamethy lenetetramine (RB/H) was fixed at RB/H = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the sintering temperatures (TS) were changed from TS = 600 °C to TS = 700 °C for t = 12 h. For the second series of BCNO phosphors, the sintering temperature was fixed at 650 °C and RB/H = 1
1, and the sintering temperatures (TS) were changed from TS = 600 °C to TS = 700 °C for t = 12 h. For the second series of BCNO phosphors, the sintering temperature was fixed at 650 °C and RB/H = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, while the heating times were varied from t = 4 h to t = 24 h. For the third series of BCNO phosphors, the sintering temperature TS = 650 °C and heating time t = 12 h, and mole ratios of boric acid (fixed at 0.02 mol) and hexamethy lenetetramine were changed from RB/H = 1
1, while the heating times were varied from t = 4 h to t = 24 h. For the third series of BCNO phosphors, the sintering temperature TS = 650 °C and heating time t = 12 h, and mole ratios of boric acid (fixed at 0.02 mol) and hexamethy lenetetramine were changed from RB/H = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 to RB/H = 1
0.1 to RB/H = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. In addition, the high temperature (T = 300–500 K) emission spectra of the BCNO phosphor synthesized with RB/H = 1
5. In addition, the high temperature (T = 300–500 K) emission spectra of the BCNO phosphor synthesized with RB/H = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, TS = 650 °C, and t = 12 h were studied.
1, TS = 650 °C, and t = 12 h were studied.
The phase structure of BCNO phosphors was characterized by powder X-ray diffraction (XRD) (Bruker Model D8). The morphology of BCNO phosphors was measured by a scanning electronic microscope (Hitachi, S-4800). X-Ray photoelectron spectroscopy (XPS, PHI1600EXCA) was used to characterize the chemical state of BCNO phosphors. The chemical bonding status was measured by Fourier transform infrared (FTIR) spectrometer (Bruker, WQF-410). The ultraviolet-visible (UV-vis) absorption spectra were measured by a spectrophotometer (Hitchi, U-3900H). The excitation spectra, emission spectra and decay curves of BCNO phosphors were measured by a steady and transient state spectrophotometer (Horiba, FL-3-22). The high temperature emission spectra in the range of T = 300–500 K were also measured by steady and transient state spectrophotometer with a high temperature attachment.
Results and discussions
Fig. S1† shows the XRD pattern and SEM image of the BCNO phosphor sintered at 650 °C for 12 h with RB/H = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. There are two broad peaks centered at 22.2° and 43.1°, which is induced by the BCNO phosphor with turbostratic boron nitride (t-BN) structure. The obvious peak at 22.2° was related to (002) reflection, and the peak around 43.1° was originated from (100) and (101) reflections, which indicated the formation of BCNO phosphors.8a In addition, there were two sharp peaks centered at 14.6° and 27.9° corresponding to (111) and (310) reflections of B2O3 with cubic structure, respectively. The cubic B2O3 was produced by the thermal decomposition of unreacted boric acid. The SEM image (inset of Fig. S1(a)†) displayed the BCNO phosphor was irregular in shape, and the particle size was in the range of several micrometers to one hundred micrometers. From the XRD and SEM results, it suggested that the prepared samples consisted of BCNO phosphor and B2O3, and the particle size was in micro scale.
1. There are two broad peaks centered at 22.2° and 43.1°, which is induced by the BCNO phosphor with turbostratic boron nitride (t-BN) structure. The obvious peak at 22.2° was related to (002) reflection, and the peak around 43.1° was originated from (100) and (101) reflections, which indicated the formation of BCNO phosphors.8a In addition, there were two sharp peaks centered at 14.6° and 27.9° corresponding to (111) and (310) reflections of B2O3 with cubic structure, respectively. The cubic B2O3 was produced by the thermal decomposition of unreacted boric acid. The SEM image (inset of Fig. S1(a)†) displayed the BCNO phosphor was irregular in shape, and the particle size was in the range of several micrometers to one hundred micrometers. From the XRD and SEM results, it suggested that the prepared samples consisted of BCNO phosphor and B2O3, and the particle size was in micro scale.
Fig. S2† displays XPS spectra and FTIR spectrum in the range of 400–2500 cm−1 for BCNO phosphor prepared at 650 °C for 12 h with RB/H = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The XPS spectra indicated the prepared phosphors were consist of B, C, N, and O elements.8b For B1s spectrum, it could be fitted well with two Gaussian curves centered at 193.0 eV and 190.9 eV, corresponding to B–O and B–N bonds, respectively,5a and no B–C signals could be observed in B1s spectrum. The C1s spectrum was fitted with three components centered at 286.2, 284.5, and 281.7 eV which were related to C
1. The XPS spectra indicated the prepared phosphors were consist of B, C, N, and O elements.8b For B1s spectrum, it could be fitted well with two Gaussian curves centered at 193.0 eV and 190.9 eV, corresponding to B–O and B–N bonds, respectively,5a and no B–C signals could be observed in B1s spectrum. The C1s spectrum was fitted with three components centered at 286.2, 284.5, and 281.7 eV which were related to C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N, C–C and C
N, C–C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively.8b–e In the N1s spectrum, the peak at 398.4 eV was originated from N–B bonds, while the shoulder peak at 399.9 eV was owing to the formation of C
O bonds, respectively.8b–e In the N1s spectrum, the peak at 398.4 eV was originated from N–B bonds, while the shoulder peak at 399.9 eV was owing to the formation of C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bonds.8c,d For the O1s spectrum, it was fitted well with only one Gaussian curve centered at 532.8 eV which was assigned to O–B bonds.8f The chemical bond states of BCNO phosphors can also be verified by FTIR spectra, as shown in Fig. S2(e).† The absorption at 785 and 1377 cm−1 was related to B–N–B vibration and B–N stretching mode, which indicated the formation of B–N linkage in BCNO phosphor.7a,8g The weak B–N stretching bands and B–N–B bands were induced by the partially crystalline BCNO phosphors.8h The B–C vibration peak was observed at 1196 cm−1, and the absorption peaks centered at 1654, 2264, 2362 cm−1 were assigned to C
N bonds.8c,d For the O1s spectrum, it was fitted well with only one Gaussian curve centered at 532.8 eV which was assigned to O–B bonds.8f The chemical bond states of BCNO phosphors can also be verified by FTIR spectra, as shown in Fig. S2(e).† The absorption at 785 and 1377 cm−1 was related to B–N–B vibration and B–N stretching mode, which indicated the formation of B–N linkage in BCNO phosphor.7a,8g The weak B–N stretching bands and B–N–B bands were induced by the partially crystalline BCNO phosphors.8h The B–C vibration peak was observed at 1196 cm−1, and the absorption peaks centered at 1654, 2264, 2362 cm−1 were assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C
C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bonds, respectively.5f,8i The vibration at 544, 644, 734, and 1448 cm−1 were originated from B–O bonds, and the absorption at 885 cm−1 corresponded to stretching vibration of tetrahedral BO4− units in B2O3 crystal.8j The N–B–O stretching bands in 900–1100 cm−1 were also observed, which was induced by the oxidation of N–B bonds.7a From the XPS and FTIR, it could be concluded that C and O elements were involved in the structure of prepared samples and bonded to B and N to form BCNO phosphors.
N bonds, respectively.5f,8i The vibration at 544, 644, 734, and 1448 cm−1 were originated from B–O bonds, and the absorption at 885 cm−1 corresponded to stretching vibration of tetrahedral BO4− units in B2O3 crystal.8j The N–B–O stretching bands in 900–1100 cm−1 were also observed, which was induced by the oxidation of N–B bonds.7a From the XPS and FTIR, it could be concluded that C and O elements were involved in the structure of prepared samples and bonded to B and N to form BCNO phosphors.
The influence of heating temperature and time on excitation and emission spectra for BCNO phosphors were investigated. Fig. 1(a) shows the normalized excitation (λem ∼ 610 nm) and emission spectra (λex = 370 nm) of BCNO phosphors synthesized with RB/H = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at different sintering temperatures for 12 h. The BCNO phosphors had wide excitation spectra range which was between 300 nm and 570 nm, and the excitation spectra range was not sensitive to the sintering temperature. There were three excitation peaks centered at 370 nm, 470 nm and 550–570 nm. The 370 nm excitation was induced by the transition from the valence band to nitrogen defects levels.9a The 470 nm and 550–570 nm excitation was originated from the transition from carbon and oxygen impurity levels to conduction bands and nitrogen defects levels, respectively. The 550–570 nm excitation peaks shifted from 550 nm to 570 nm first with increasing heating temperature from TS = 600 °C to 650 °C and then the excitation peaks shifted back to 554 nm with further increasing sintering temperature. The shift of excitation peaks was related to the energy levels' position which was originated form carbon and oxygen impurity defects. With increasing temperature, the position of carbon and oxygen related impurity levels was changed and led to the shift of excitation peaks, which could be reflected by UV-vis absorption spectra. In addition, the relative excitation peak intensity of 550–570 nm was increased first and then decreased with the enhancement of sintering temperature, which might be resulted from the varied concentration of carbon and oxygen impurity. For the emission spectra, the emission peak position shifted from 465 nm for TS = 600 °C to 620 nm for TS = 650 °C, and then the emission peak shifted to 602 nm with further increasing temperature. For the low sintering temperature (TS = 600 °C), BCNO phosphors were not completely formed, and the luminescence is dominated by blue emission centered at 470 nm. However, a shoulder emission peak in red emission range was also observed on the emission spectra for the BCNO phosphor. With increasing temperature, the blue emission intensity was decreased rapidly and the red emission became the dominate luminescence center in BCNO phosphors. The red emission intensity of BCNO phosphors was increased with heating temperature first and reached a maximum value at 675 °C and then was decreased, which may be related to the crystallinity of BCNO phosphors. The slightly emission peak shift from 620 nm to 602 nm was induced by the change of carbon and oxygen impurity concentration and the shift of the impurity levels with increasing temperature.5c The luminescence spectra of BCNO phosphors were also affected by heating time. Fig. 1(b) displays the normalized excitation and emission spectra of BCNO phosphors with RB/H = 1
1 at different sintering temperatures for 12 h. The BCNO phosphors had wide excitation spectra range which was between 300 nm and 570 nm, and the excitation spectra range was not sensitive to the sintering temperature. There were three excitation peaks centered at 370 nm, 470 nm and 550–570 nm. The 370 nm excitation was induced by the transition from the valence band to nitrogen defects levels.9a The 470 nm and 550–570 nm excitation was originated from the transition from carbon and oxygen impurity levels to conduction bands and nitrogen defects levels, respectively. The 550–570 nm excitation peaks shifted from 550 nm to 570 nm first with increasing heating temperature from TS = 600 °C to 650 °C and then the excitation peaks shifted back to 554 nm with further increasing sintering temperature. The shift of excitation peaks was related to the energy levels' position which was originated form carbon and oxygen impurity defects. With increasing temperature, the position of carbon and oxygen related impurity levels was changed and led to the shift of excitation peaks, which could be reflected by UV-vis absorption spectra. In addition, the relative excitation peak intensity of 550–570 nm was increased first and then decreased with the enhancement of sintering temperature, which might be resulted from the varied concentration of carbon and oxygen impurity. For the emission spectra, the emission peak position shifted from 465 nm for TS = 600 °C to 620 nm for TS = 650 °C, and then the emission peak shifted to 602 nm with further increasing temperature. For the low sintering temperature (TS = 600 °C), BCNO phosphors were not completely formed, and the luminescence is dominated by blue emission centered at 470 nm. However, a shoulder emission peak in red emission range was also observed on the emission spectra for the BCNO phosphor. With increasing temperature, the blue emission intensity was decreased rapidly and the red emission became the dominate luminescence center in BCNO phosphors. The red emission intensity of BCNO phosphors was increased with heating temperature first and reached a maximum value at 675 °C and then was decreased, which may be related to the crystallinity of BCNO phosphors. The slightly emission peak shift from 620 nm to 602 nm was induced by the change of carbon and oxygen impurity concentration and the shift of the impurity levels with increasing temperature.5c The luminescence spectra of BCNO phosphors were also affected by heating time. Fig. 1(b) displays the normalized excitation and emission spectra of BCNO phosphors with RB/H = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 sintered at 650 °C for different heating times. For t = 4 h, there were two emission peaks centered at 473 nm and 605 nm, which was induced by the partially crystal BCNO phosphor. With increasing time, the amount of BCNO was increased and the crystallinity of BCNO phosphor was improved, which led to the disappearance of blue emission and the appearance of dominated red emission. The intensity of red emission peak was enhanced with increasing heating time from t = 4 h to t = 12 h and then it was decreased with further extending heating time. The red emission peak was slightly shifted from 622 nm for t = 8 h to 611 nm for t = 24 h, which indicated the carbon and oxygen impurity levels' position was not very sensitive to longer heating time. The blue shift of emission peak might be induced by the change of optical band gap, which could be reflected from absorption spectra and optical band gap value. The range and tendency of excitation spectra for BCNO phosphor with different sintering times were very similar to that of BCNO phosphors prepared with different sintering temperatures.
1 sintered at 650 °C for different heating times. For t = 4 h, there were two emission peaks centered at 473 nm and 605 nm, which was induced by the partially crystal BCNO phosphor. With increasing time, the amount of BCNO was increased and the crystallinity of BCNO phosphor was improved, which led to the disappearance of blue emission and the appearance of dominated red emission. The intensity of red emission peak was enhanced with increasing heating time from t = 4 h to t = 12 h and then it was decreased with further extending heating time. The red emission peak was slightly shifted from 622 nm for t = 8 h to 611 nm for t = 24 h, which indicated the carbon and oxygen impurity levels' position was not very sensitive to longer heating time. The blue shift of emission peak might be induced by the change of optical band gap, which could be reflected from absorption spectra and optical band gap value. The range and tendency of excitation spectra for BCNO phosphor with different sintering times were very similar to that of BCNO phosphors prepared with different sintering temperatures.
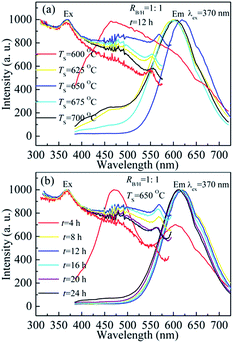 | ||
Fig. 1 Normalized excitation and emission spectra of BCNO phosphors prepared with (a) different sintering temperatures and (b) various heating times with RB/H = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
Fig. 2 shows the normalized excitation and emission spectra of BCNO phosphors synthesized at 650 °C for 12 h with different ratios of boric acid and hexamethy lenetetramine (RB/H = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1–1
0.1–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). For RB/H = 1
5). For RB/H = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, the emission of BCNO phosphor was dominated by blue emitting centered at 410 nm and 445 nm, which was induced by the carbon doped B2O3 that was similar to carbon doped Al2O3.9b In addition, the excitation spectra was in the range of ultraviolet and centered at 275 nm, which was induced by the transition of carbon impurity levels to conduction band. With increasing content of hexamethy lenetetramine (RB/H = 1
0.1, the emission of BCNO phosphor was dominated by blue emitting centered at 410 nm and 445 nm, which was induced by the carbon doped B2O3 that was similar to carbon doped Al2O3.9b In addition, the excitation spectra was in the range of ultraviolet and centered at 275 nm, which was induced by the transition of carbon impurity levels to conduction band. With increasing content of hexamethy lenetetramine (RB/H = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3), the boric acid become not too much and more BCNO phosphors were formed, which led to the decrease of blue emission and increase of red emission intensity. With further increasing hexamethy lenetetramine (RB/H ≥ 1
0.3), the boric acid become not too much and more BCNO phosphors were formed, which led to the decrease of blue emission and increase of red emission intensity. With further increasing hexamethy lenetetramine (RB/H ≥ 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5), the blue emission was disappeared and there was only red emission of BCNO phosphors. The red emission peak position was shifted from 597 nm for RB/H = 1
0.5), the blue emission was disappeared and there was only red emission of BCNO phosphors. The red emission peak position was shifted from 597 nm for RB/H = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 to 620 nm for RB/H = 1
0.5 to 620 nm for RB/H = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and then the red emission peak took a blue shift to 590 nm for RB/H = 1
1, and then the red emission peak took a blue shift to 590 nm for RB/H = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 sample. The red emission intensity was increased first and then it started to decrease at RB/H = 1
5 sample. The red emission intensity was increased first and then it started to decrease at RB/H = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, which might be related to the concentration and band structure of BCNO phosphors. The shift of emission spectra with increasing RB/H was induced by the change of carbon related impurity levels' position. For RB/H ≤ 1
3, which might be related to the concentration and band structure of BCNO phosphors. The shift of emission spectra with increasing RB/H was induced by the change of carbon related impurity levels' position. For RB/H ≤ 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the carbon impurity levels would shift towards to conduction bands with increasing carbon impurity concentration, which led to the red shift of emission spectra. For RB/H > 1
1, the carbon impurity levels would shift towards to conduction bands with increasing carbon impurity concentration, which led to the red shift of emission spectra. For RB/H > 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, there were too much carbon impurity concentration and formation of carbon residue on the product might cause the blue shift of emission spectra.6a The excess carbon was not effectively doped in BCNO phosphors and the floating carbon impurity on surface of BCNO phosphors had negative effects on red emission of BCNO phosphors, which might lead to the blue shift of emission spectra for too much carbon. For excitation spectra, there were also three excitation peaks centered at 370, 470 and 533–570 nm for RB/H ≥ 1
1, there were too much carbon impurity concentration and formation of carbon residue on the product might cause the blue shift of emission spectra.6a The excess carbon was not effectively doped in BCNO phosphors and the floating carbon impurity on surface of BCNO phosphors had negative effects on red emission of BCNO phosphors, which might lead to the blue shift of emission spectra for too much carbon. For excitation spectra, there were also three excitation peaks centered at 370, 470 and 533–570 nm for RB/H ≥ 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3. The relative excitation peak intensity for 470 nm and 533–570 nm were increased from RB/H = 1
0.3. The relative excitation peak intensity for 470 nm and 533–570 nm were increased from RB/H = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 to RB/H = 1
0.3 to RB/H = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and then the relative excitation intensity was reduced with the further increase of RB/H. The excitation peak position at 533–570 nm was also related to the change of carbon related impurity levels' position.
1, and then the relative excitation intensity was reduced with the further increase of RB/H. The excitation peak position at 533–570 nm was also related to the change of carbon related impurity levels' position.
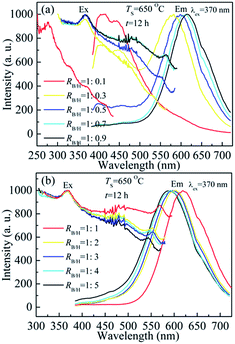 | ||
Fig. 2 Normalized excitation spectra and emission spectra of BCNO phosphors prepared at 650 C for 12 h with (a) RB/H = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1–1 0.1–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9 and (b) RB/H = 1 0.9 and (b) RB/H = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–1 1–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. 5. | ||
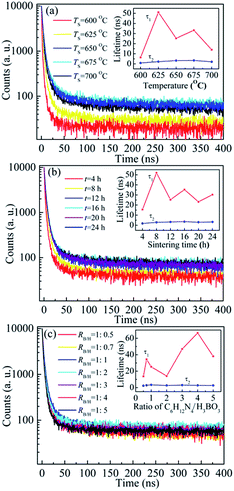
We also investigated the effects of preparation conditions on life times for BCNO phosphors. Fig. 3 displays the red emission peak decay curves of BCNO phosphors prepared with different sintering temperatures (TS = 600–700 °C) and times (t = 4–24 h), and various ratios of boric acid and hexamethy lenetetramine (RB/H = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5–1
0.5–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). All the decay curves can be fitted well by dual exponential function as the formula:9c
5). All the decay curves can be fitted well by dual exponential function as the formula:9c
I(t) = I1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ1) + I2 exp(−t/τ1) + I2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ2) exp(−t/τ2)
| (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5–1
0.5–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, the τ1 of BCNO phosphors was changed between 13.7 ns and 66.5 ns, and τ2 was varied in the range of 2.3–3.3 ns. There were two lifetime peaks appeared at τ1 = 34.4 ns with RB/H = 1
5, the τ1 of BCNO phosphors was changed between 13.7 ns and 66.5 ns, and τ2 was varied in the range of 2.3–3.3 ns. There were two lifetime peaks appeared at τ1 = 34.4 ns with RB/H = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 and τ1 = 66.5 ns with RB/H = 1
0.5 and τ1 = 66.5 ns with RB/H = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, respectively. The change of life times τ1 and τ2 with preparing condition might be related to the carbon and oxygen impurity concentration during the formation of BCNO phosphors.5c From the decay curves and fitting results of τ1 and τ2 for BCNO phosphors, it could be concluded that the red emission was induced by two luminescence centers, corresponding to longer lifetime τ1 and short lifetime τ2. The two luminescence centers could also be verified by red emission spectra of BCNO phosphors, there was an obvious shoulder emission peak on red emission spectra and the red emission spectra could be fitted well by two Gaussian curves (not shown).
4, respectively. The change of life times τ1 and τ2 with preparing condition might be related to the carbon and oxygen impurity concentration during the formation of BCNO phosphors.5c From the decay curves and fitting results of τ1 and τ2 for BCNO phosphors, it could be concluded that the red emission was induced by two luminescence centers, corresponding to longer lifetime τ1 and short lifetime τ2. The two luminescence centers could also be verified by red emission spectra of BCNO phosphors, there was an obvious shoulder emission peak on red emission spectra and the red emission spectra could be fitted well by two Gaussian curves (not shown).
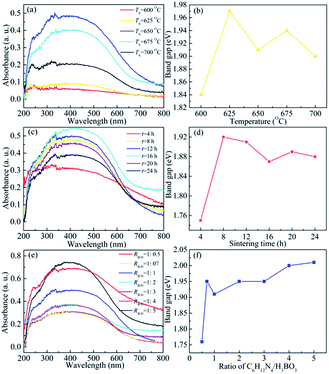
The UV-vis absorption spectra and optical band gaps of BCNO phosphors prepared with different sintering temperatures and times, and various ratios of boric acid and hexamethy lenetetramine were shown in Fig. 4. The optical band gap values were calculated by the relation as below:9d
| αhν = A(hν − Eg)1/2 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 to 1.95 eV for RB/H = 1
0.5 to 1.95 eV for RB/H = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7, and then it was increased slowly to 2.01 eV for RB/H = 1
0.7, and then it was increased slowly to 2.01 eV for RB/H = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. The first quickly enhancement of band gap was related to the increase content of BCNO phosphors, and the slowly increase of band gap with RB/H was resulted from the change of carbon and nitrogen related defects' levels position. For smaller ratio of boric acid to hexamethy lenetetramine, the carbon impurity concentration was higher and their related levels were shifted towards conduction band, which resulted in a smaller band gap value. With increasing RB/H, the relative carbon concentration was decreased and their impurity levels were shifted away from conduction band, which led to the increase of optical ban gap value. The broad absorption of BCNO phosphors in visible light range might be used in solar cell to improve its light absorption and conversion efficiency.
5. The first quickly enhancement of band gap was related to the increase content of BCNO phosphors, and the slowly increase of band gap with RB/H was resulted from the change of carbon and nitrogen related defects' levels position. For smaller ratio of boric acid to hexamethy lenetetramine, the carbon impurity concentration was higher and their related levels were shifted towards conduction band, which resulted in a smaller band gap value. With increasing RB/H, the relative carbon concentration was decreased and their impurity levels were shifted away from conduction band, which led to the increase of optical ban gap value. The broad absorption of BCNO phosphors in visible light range might be used in solar cell to improve its light absorption and conversion efficiency.
To further investigated the luminescence properties and thermal stability of red emitting BCNO phosphors, the high temperature emission spectra of BCNO phosphor were measured between T = 300 K and T = 500 K, as shown in Fig. 5. Fig. 5(a) shows the high temperature emission spectra excited by 370 nm for BCNO phosphor synthesized at 650 °C for 12 h with RB/H = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The red emission peak position was not influenced by the enhancement of temperature, which indicated that the transition levels' position was not changed by the sample temperature. However, the emission peak intensity was affected by the sample temperature, as shown in Fig. 5(b). The emission peak intensity was slightly decreased for T ≤ 400 K. With enhancing sample temperature, the emission peak intensity was increased and reached a maximum value at T = 440 K (∼170 °C), and then the emission peak intensity started to reduce with increasing sample temperature. The tendency of red emission peak intensity under various sample temperatures was correlated with the luminescence process of the BCNO phosphors. In BCNO phosphors, carbon related impurity was mainly served as donor impurity levels which provided electrons in the transition process.2c,9d,10a The carbon related impurity levels might shift towards to conduction band with increasing carbon related impurity concentration. In addition, there were lots of nitrogen defects such as nitrogen vacancies in BCNO phosphors. The nitrogen vacancies could be served as electron traps and their defect levels were located at 0.7–1.0 eV below the conduction band.10b,c For 370 nm (∼3.4 eV) excitation, the electrons would be excited from carbon related impurity levels to conduction band. Then the electrons would jump to nitrogen vacancy levels. Some electrons would be trapped by nitrogen vacancies, and some electrons would jump from the nitrogen vacancy levels to carbon related impurity levels. The red emission of BCNO phosphors was induced by the transition between nitrogen vacancy levels and carbon related impurity levels (∼2.0 eV). For low heating temperatures (T < 410 K), the electron trapping and electron transition kept a fine equilibrium. With increasing temperature (410 K ≤ T ≤ 440 K), the equilibrium was destructed and the trapped electrons would also attend the transition from nitrogen vacancy levels to carbon related impurity levels, which resulted in the enhancement of emission intensity. With further increasing temperature (T > 440 K), the nitrogen vacancy was increased and there was no enough trapped electrons to attend the transition, then the excited electrons would be trapped first and less electrons would jump from nitrogen vacancy levels to carbon related impurity levels, which led to the decrease of emission intensity. In addition, the lattice relaxation and non radiation process would increase under higher temperature, which would also reduce the red emission intensity of BCNO phosphor.10d From Fig. 5(b), it can also be seen that the BCNO phosphor keep high emission intensity without decreasing for T ≤ 480 K (∼210 °C), which indicates the BCNO phosphors have good thermal stability and it would be beneficial for application.10e
1. The red emission peak position was not influenced by the enhancement of temperature, which indicated that the transition levels' position was not changed by the sample temperature. However, the emission peak intensity was affected by the sample temperature, as shown in Fig. 5(b). The emission peak intensity was slightly decreased for T ≤ 400 K. With enhancing sample temperature, the emission peak intensity was increased and reached a maximum value at T = 440 K (∼170 °C), and then the emission peak intensity started to reduce with increasing sample temperature. The tendency of red emission peak intensity under various sample temperatures was correlated with the luminescence process of the BCNO phosphors. In BCNO phosphors, carbon related impurity was mainly served as donor impurity levels which provided electrons in the transition process.2c,9d,10a The carbon related impurity levels might shift towards to conduction band with increasing carbon related impurity concentration. In addition, there were lots of nitrogen defects such as nitrogen vacancies in BCNO phosphors. The nitrogen vacancies could be served as electron traps and their defect levels were located at 0.7–1.0 eV below the conduction band.10b,c For 370 nm (∼3.4 eV) excitation, the electrons would be excited from carbon related impurity levels to conduction band. Then the electrons would jump to nitrogen vacancy levels. Some electrons would be trapped by nitrogen vacancies, and some electrons would jump from the nitrogen vacancy levels to carbon related impurity levels. The red emission of BCNO phosphors was induced by the transition between nitrogen vacancy levels and carbon related impurity levels (∼2.0 eV). For low heating temperatures (T < 410 K), the electron trapping and electron transition kept a fine equilibrium. With increasing temperature (410 K ≤ T ≤ 440 K), the equilibrium was destructed and the trapped electrons would also attend the transition from nitrogen vacancy levels to carbon related impurity levels, which resulted in the enhancement of emission intensity. With further increasing temperature (T > 440 K), the nitrogen vacancy was increased and there was no enough trapped electrons to attend the transition, then the excited electrons would be trapped first and less electrons would jump from nitrogen vacancy levels to carbon related impurity levels, which led to the decrease of emission intensity. In addition, the lattice relaxation and non radiation process would increase under higher temperature, which would also reduce the red emission intensity of BCNO phosphor.10d From Fig. 5(b), it can also be seen that the BCNO phosphor keep high emission intensity without decreasing for T ≤ 480 K (∼210 °C), which indicates the BCNO phosphors have good thermal stability and it would be beneficial for application.10e
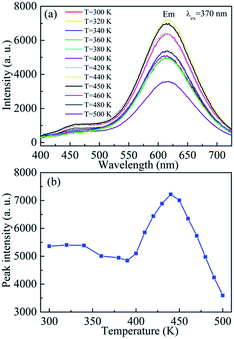 | ||
Fig. 5 (a) High temperature emission spectra of BCNO phosphors sintered at 650 °C for 12 h with RB/H = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. (b) Emission peak intensity as a function of sample temperature with T = 300–500 K. 1. (b) Emission peak intensity as a function of sample temperature with T = 300–500 K. | ||
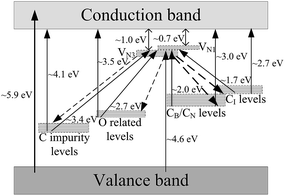
Based on the above results and our previous research, a simplified energy level diagram was tentatively constructed to explain the luminescence mechanism of BCNO phosphors, as shown in Fig. 6. With no C and O doping, BN has a large band gap with about 5.9 eV. The nitrogen vacancy levels called three boron center (VN3) and one-boron center (VN1) will be formed below conduction band 1.0 and 0.7 eV, respectively.9c,d For less C and O doping and partially formed BCNO, carbon and oxygen levels will appear in the band gap below the conduction band 4.1 and 3.5 eV, respectively.6d,8h For more C and O doping and completely formed BCNO phosphor, new carbon related levels such as CB (C substituted B) or CN (C substituted N) levels will appear below the conduction band 3.0 eV.5g,8d For partially formed BCNO phosphors, the excitation spectra and absorption spectra were induced by the transition from valence band to conduction band (λex = 210 nm to 5.9 eV), valance band to nitrogen vacancy levels (λex = 270 nm to 4.6 eV), carbon impurity levels (such as C substituted O levels) to conduction band (λex = 270 nm to 4.1 eV), oxygen energy levels to conduction band (λex = 354 nm to 3.5 eV). After relaxation, the photon electrons will be trapped by nitrogen vacancy levels, and the emission spectra were mainly in blue light range and originated from the transition from nitrogen vacancy levels to carbon impurity levels (λem = 400 nm to 3.1 eV) and oxygen levels (λem = 460 nm to 2.7 eV), respectively. In addition, the weak red emission was induced by the transition from vacancy levels to CB or CN levels (λem = 600 nm to 2.0 eV). For completely formed BCNO phosphors, the blue excitation around 460 nm was induced by the transition from CB, CN and CI (interstitial carbon) levels to conduction band (λex = 460 nm to 2.7 eV). The excitation around 550–580 nm was originated from the transition from CB, CN and CI levels to nitrogen vacancy levels (λex = 550–580 nm to 2.1–2.3 eV). The red emission of BCNO phosphors was dominated by the transition from nitrogen vacancy levels to CB, CN and CI levels (λem = 600–620 nm to 2.0 eV). In addition, the broad absorption spectra between 210 and 700 nm (∼1.7–5.9 eV) were induced by the transition from valance band, carbon and oxygen related levels to conduction band and nitrogen vacancy levels.
Conclusions
In summary, red emitting BCNO phosphors were successfully synthesized by solid state reaction method and the spectral properties and luminescence mechanism were systematically investigated. The red emission BCNO phosphors were turbostratic boron nitride structure and their size was in the range of several micrometers to one hundred micrometers. The XPS spectra and FTIR spectra indicated the carbon and oxygen elements were bonded with boron and nitrogen elements. The excitation spectra of BCNO phosphors were in the range of 300–570 nm, and the emission spectra were shifted from blue light to red light with increasing sintering temperature, time and the ratio of boric acid to hexamethy lenetetramine, corresponding with the partially formed BCNO to completely formed BCNO phosphor. The shift of emission spectra was resulted from the change of carbon and oxygen related impurity concentration and appearance of new carbon related impurity (CB/CN/CI) levels. The decay curves suggested that the red emission process were induced by two luminescence centers with short and long lifetimes, respectively. The short lifetime was not sensitive to preparation conditions while the long lifetime was varied with sintering temperature, time and the mole ratio of raw materials. The UV-vis absorption spectra disclosed that the red emitting BCNO phosphors had wide absorption range (210–700 nm) which was originated from the transition from valence band, carbon and oxygen related levels to conduction band and nitrogen vacancy levels. In addition, the high temperature red emission spectra of BCNO phosphor implied that the nitrogen vacancies served as electron trapping which would participate in the red emitting process under certain temperatures (T > 410 K). The red emitting BCNO phosphors with good stability have great potential application on white LEDs, solar cell, display and biomedical fields.Acknowledgements
This work was supported by the National Natural Science Foundation of China (nos 51202055, 51402084, 51172060, 51171056, 51371075, 51272064) and Natural Science Foundation of Hebei Province of China (no. E2014202131), and Excellent Youth Foundation for Institution of Higher Learning of Hebei Province (no. YQ2014017). We also appreciate Dr Jianping Xu for the measurement and discussion of high temperature photoluminescence spectra.Notes and references
- (a) S. E. Brinkley, N. Pfaff, K. A. Denault, Z. Zhang, H. T. Hintzen, R. Seshadri, S. Nakamura and S. P. DenBaars, Appl. Phys. Lett., 2011, 99, 241106 CrossRef PubMed; (b) L. Yang, N. Zhang, R. Y. Zhang, B. Wen, H. L. Li and X. B. Bian, Mater. Lett., 2014, 129, 134 CrossRef CAS PubMed; (c) B. Wang, H. Lin, J. Xu, H. Chen and Y. S. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 22905 CrossRef CAS PubMed; (d) B. Mahler, N. Lequeux and B. Dubertret, J. Am. Chem. Soc., 2010, 132, 953 CrossRef CAS PubMed.
- (a) K. Watanabe, T. Taniguchi and H. Kanda, Nat. Mater., 2004, 3, 404 CrossRef CAS PubMed; (b) Y. Kubota, K. Watanabe, O. Tsuda and T. Taniguchi, Science, 2007, 317, 932 CrossRef CAS PubMed; (c) L. Museur, E. Feldbach and A. Kanaev, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 155204 CrossRef.
- (a) A. Rubio, J. L. Corkill and M. L. Cohen, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49, 5081 CrossRef CAS; (b) M. O. Watanabe, S. Itoh, T. Sasaki and K. Mizushima, Phys. Rev. Lett., 1996, 77, 187 CrossRef CAS; (c) C. Y. Zhi, X. D. Bai and E. G. Wang, Appl. Phys. Lett., 2002, 80, 3590 CrossRef CAS PubMed; (d) C. Y. Zhi, X. D. Bai and E. G. Wang, J. Nanosci. Nanotechnol., 2004, 4, 35 CrossRef CAS PubMed.
- (a) T. Ogi, Y. Kaihatsu, F. Iskandar, W. N. Wang and K. Okuyama, Adv. Mater., 2008, 20, 3235 CrossRef CAS PubMed; (b) W. N. Wang, T. Ogi, Y. Kaihatsu, F. Iskandar and K. Okuyama, J. Mater. Chem., 2011, 21, 5183 RSC.
- (a) L. Singh and V. Chopra, J. Mater. Sci. Technol., 2011, 27, 967 CrossRef CAS; (b) W. W. Lei, D. Portehault, R. Dimova and M. Antonietti, J. Am. Chem. Soc., 2011, 133, 7121 CrossRef CAS PubMed; (c) X. H. Zhang, Z. M. Lu, J. Lin, Y. Fan, L. L. Li, X. W. Xu, L. Hu, F. B. Meng, J. L. Zhao and C. C. Tang, ECS J. Solid State Sci. Technol., 2013, 2, R39 CrossRef CAS PubMed; (d) C. R. Ren, X. H. Zhang, L. Y. Zhou, Z. M. Lu, J. Lin, X. W. Xu, L. L. Li, X. Zhang, Y. M. Xue, F. B. Meng, J. L. Zhao and C. C. Tang, J. Lumin., 2014, 153, 338 CrossRef CAS PubMed; (e) X. H. Zhang, Z. M. Lu, J. Lin, L. L. Li, Y. Fan, L. Hu, X. W. Xu, F. B. Meng, J. L. Zhao and C. C. Tang, Mater. Lett., 2013, 94, 72 CrossRef CAS PubMed; (f) A. B. Suryamas, M. M. Munir, T. Ogi, Khairurrijal and K. Okuyama, J. Mater. Chem., 2011, 21, 12629 RSC; (g) Q. H. Weng, X. B. Wang, X. Wang, D. Q. Liu, X. F. Jiang, C. Y. Zhi, Y. Bando and D. Golberg, Chem.–Asian J., 2013, 8, 2936 CrossRef CAS PubMed.
- (a) Y. Kaihatsu, W. N. Wang, F. Iskandar, T. Ogi and K. Okuyama, J. Electrochem. Soc., 2010, 157, J329 CrossRef CAS PubMed; (b) T. Ogi, F. Iskandar, A. B. D. Nandiyanto, W. N. Wang and K. Okuyama, J. Chem. Eng. Jpn., 2012, 45, 995 CrossRef CAS; (c) F. Lu, X. H. Zhang, Z. M. Lu, X. W. Xu and C. C. Tang, J. Lumin., 2013, 143, 343 CrossRef CAS PubMed; (d) Z. Y. Chu, Y. Kang, Z. H. Jiang, G. Y. Li, T. J. Hu, J. Wang, Z. F. Zhou, Y. H. Li and X. J. Wang, RSC Adv., 2014, 4, 26855 RSC.
- (a) C. C. Tang, Y. Bando, C. Y. Zhi and D. Golberg, Chem. Commun., 2007, 44, 4599 RSC; (b) Y. Kaihatsu, F. Iskandar, H. Widiyandari, W. N. Wang and K. Okuyama, Electrochem. Solid-State Lett., 2009, 12, J33 CrossRef CAS PubMed; (c) X. F. Liu, S. Ye, G. P. Dong, Y. B. Qiao, J. Ruan, Y. X. Zhuang, Q. Zhang, G. Lin, D. P. Chen and J. R. Qiu, J. Phys. D: Appl. Phys., 2009, 42, 215409 CrossRef.
- (a) X. F. Liu, S. Ye, Y. B. Qiao, G. P. Dong, Q. Zhang and J. R. Qiu, Chem. Commun., 2009, 27, 4073 RSC; (b) T. Ogi, H. Iwasaki, A. B. D. Nadiyanto, F. Iskandar, W. N. Wang and K. Okuyama, J. Mater. Chem. C, 2014, 2, 4297 RSC; (c) L. J. Ci, L. Song, C. H. Jin, D. Jariwala, D. X. Wu, Y. J. Li, A. Srivastava, Z. F. Wang, K. Storr, L. Balicas, F. Liu and P. M. Ajayan, Nat. Mater., 2010, 9, 430 CrossRef CAS PubMed; (d) K. Raidongia, A. Nag, K. P. S. S. Hembram, U. V. Waghmare, R. Datta and C. N. R. Rao, Chem.–Eur. J., 2010, 16, 149 CrossRef CAS PubMed; (e) Z. Bastl and S. Černý, J. Alloys Compd., 1991, 176, 159 CrossRef CAS; (f) C. Y. Zhi, Y. Bando, C. C. Tang, D. Golberg, R. G. Xie and T. Sekigushi, Appl. Phys. Lett., 2005, 86, 213110 CrossRef PubMed; (g) X. Gouin, P. Grange, L. Bois, P. L'Haridon and Y. Laurent, J. Alloys Compd., 1995, 224, 22 CrossRef CAS; (h) X. H. Zhang, S. Yan, Y. H. Cheng, K. H. Gao, Z. M. Lu, F. B. Meng, J. Lin, X. W. Xu, J. L. Zhao and C. C. Tang, Mater. Lett., 2013, 102–103, 102 CrossRef CAS PubMed; (i) Z. M. El-Bahy, A. I. Hanafy, M. M. Ibrahim and M. Anpo, J. Mol. Catal. A: Chem., 2011, 344, 111 CrossRef CAS PubMed; (j) D. Singh, K. Singh, G. Singh, Manupriya, S. Mohan, M. Arora and G. Sharma, J. Phys.: Condens. Matter, 2008, 20, 075228 CrossRef.
- (a) X. H. Zhang, L. L. Li, Z. M. Lu, J. Lin, X. W. Xu, Y. H. Ma, X. J. Yang, F. B. Meng, J. L. Zhao and C. C. Tang, J. Am. Ceram. Soc., 2014, 97, 246 CrossRef CAS PubMed; (b) Y. Kaihatsu, W. N. Wang, F. Iskandar and K. Okuyama, Mater. Lett., 2010, 64, 836 CrossRef CAS PubMed; (c) H. F. Li, R. Zhao, Y. L. Jia, W. Z. Sun, J. P. Fu, L. H. Jiang, S. Zhang, R. Pang and C. Y. Li, ACS Appl. Mater. Interfaces, 2014, 6, 3163 CrossRef CAS PubMed; (d) M. Girtan and G. Folcher, Surf. Coat. Technol., 2003, 172, 242 CrossRef CAS.
- (a) W. N. Wang, Y. Kaihatsu, F. Iskandar and K. Okuyama, Mater. Res. Bull., 2009, 44, 2099 CrossRef CAS PubMed; (b) A. Katzir, J. T. Suss, A. Zunger and A. Halperin, Phys. Rev. B: Condens. Matter Mater. Phys., 1975, 11, 2370 CrossRef CAS; (c) E. Y. Andrei, A. Katzir and J. T. Suss, Phys. Rev. B: Condens. Matter Mater. Phys., 1976, 13, 2831 CrossRef CAS; (d) R. J. Xie, N. Hirosaki, N. Kimura, K. Sakuma and M. Mitomo, Appl. Phys. Lett., 2007, 90, 191101 CrossRef PubMed; (e) X. J. Zhang, L. Huang, F. J. Pan, M. M. Wu, J. Wang, Y. Chen and Q. Su, ACS Appl. Mater. Interfaces, 2014, 6, 2709 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: XRD pattern, SEM, XPS and FTIR spectral of the BCNO phosphor. See DOI: 10.1039/c5ra07054f |
| This journal is © The Royal Society of Chemistry 2015 |