Synergic effects of a protic ionic liquid on P123 mixed micelles for inducing hierarchical porous materials
Shuo Zhao,
Xiaoli Sheng,
Yuming Zhou*,
Man He*,
Xiaoqin Fu and
Yiwei Zhang
School of Chemistry and Chemical Engineering, Southeast University, Jiangsu Optoelectronic Functional Materials and Engineering Laboratory, Nanjing 211189, P. R. China. E-mail: ymzhou@seu.edu.cn; Fax: +86 25 52090617; Tel: +86 25 52090617
First published on 12th June 2015
Abstract
This research reports a bimodal templating method to synthesize hierarchical porous silicas (HPS) by using P123 and a protic ionic liquid in aqueous systems. The influence of the pH, the hydrothermal temperature, and the synthesis route on the synthesis of HPS was systematically investigated. The structures of all the composites were characterized by using N2 gas sorption, scanning electron microscope (SEM) and transmission electron microscopy (TEM). The results showed that HPS with different structures were obtained under the different conditions. Among the obtained silica materials, HPS with two kinds of mesoporous channels could be clearly identified from the TEM images when the pH was 2. While, at pH = 3, it could be found that a particular structure like the mesocellular foams was obtained. Silica spheres were synthesized when the pH was 9, indicating that the different interaction between P123 and PIL under different pH values can promote the condensation of hydrolyzed silica species from TEOS into silica materials in different ways, resulting in different morphologies and internal structures. Moreover, the formation mechanism of the hierarchical porous silica which is based on the interaction between PIL and P123 is tentatively elucidated. 12-Tungstophosphoric acid (HPW) catalysts incorporated into these HPS were prepared by impregnation, and their catalytic activities for alkylation of o-xylene with styrene were investigated. Alkylation results exhibited that both of the catalysts showed a high catalytic performance in terms of propane conversion and selectivity to propene. This approach provides a new pathway for the facile synthesis of HPS materials which may be a type of promising candidate for various applications.
1. Introduction
Owing to their unique properties, such as high surface area, large pore volumes, high thermal, hydrothermal and mechanical stability1,2 and various frameworks, hierarchical porous silicas (HPS) have attracted much interest over the past few years and are applied in a variety of fields, including catalysis,3–5 adsorbents,6–8 and drug delivery carriers.9 During the past decades, various methods have been utilized to synthesize HPS, including a postsynthetic demetalation process and template synthesis.10,11 The other parameters can also be altered and controlled in the different methods. Therefore, an appropriate synthesis method is necessary to obtain the desired material. According to our knowledge, the cosolvents including cosurfactants and oils are usually used as the expanding agents for swelling the surfactant to synthesize the large pore size silica materials.12,13 Moreover, the approach of using templates has been treated as one of the most promising ways to obtain hierarchical porous silica materials as well.14 Thus, it is crucial to choose the right template due to the importance of the interaction between templates and siliceous precursors.Recently, ionic liquids (ILs), which are molten salts at room temperature, have attracted considerable attention due to their superior physical properties such as low vapor pressure, mild reaction condition, solvating ability, easy recycle ability and so on.15–17 And ILs were actively studied in all kinds of fields, such as chemistry, organometallic, biocatalyzed reactions.18,19 It is said that ILs can act as multiple roles of cosolvent, cosurfactant as well as salt in the system. Therefore, ILs have a potential to synthesize the HPS.20,21 Hu et al.22 synthesized a high-quality cubic gyroid mesoporous silica (MCM-41) with the ionic liquid 1-hexadecyl-3-methylimidazolium bromide as the template under basic condition. Gao et al.23 successfully synthesized the micro/mesoporous silicate materials using P123 and imidazolium ILs ([Cnmim]X). They investigated the influence of the alkyl chain length, the different types of anions, and the hydrophobicity of [Cnmim]X to the structure of final materials. And all the samples had a kind of ordered mesoporous channels with disordered micropores.
Mesostructured cellular foams which are part of HPS can be prepared in acidic medium with a similar way to synthesize SBA-15. In the previous work, 1,3,5-trimethylbenzene (TMB) was usually acted as a pore expander to obtain mesostructured cellular foams with large pore size and pore volume.24 And the four component microemulsion systems of surfactant/salt/oil/water were used to synthesize HPS.25,26 Zhou et al.27 obtained hierarchical porous silicas by using a P123/cosurfactant/1,3,5-trimethylbenzene/water four component microemulsion system. In their work, the HPS materials with pore structures of disordered-SBA-15-type together with mesocellular foams were formed, owing to the fact that diols and TMB could swell the P123 micelles. An et al.28 synthesized the mesocellular siliceous foam by using TMB and nonionic block copolymer surfactant Pluronic P123 under neutral pH conditions. Similarly, Schmidt-Winkel, et al.13 successfully obtained the siliceous mesostructured cellular foams with P123 and 1,3,5-trimethylbenzene (TMB). However, there are few reports on the synthesis of HPS materials by using protic ionic liquids (PIL) which are formed via the proton transfer from a Bronsted acid to a Bronsted base. It is said that many PILs are miscible with water to form mixtures at any composition, and these ions favorably form hydrogen bonds with water.29–31
In this work, HPS were synthesized with P123 and PIL (triethylamine acetate) as the co-templates by the hydrothermal method. A series of samples were synthesized by changing the synthesis route, the temperature and the pH of the solution during synthesis. The results showed that protic ionic liquid was a suitable cosurfactant for synthesizing HPS.
2. Experimental section
2.1 Chemicals
P123 (PEO20PPO70PEO20) was purchased from Sigma-Aldrich. The inorganic silica precursor was silicon(IV) tetraacetate (TEOS 97%, Fluka) and HCl (37% in water, Aldrich) was used as reaction catalyst, triethylamine (Merck) and acetic acid (Merck) were used to synthesise PIL.2.2 Preparation of PIL
Triethylamine acetate was obtained according to the literature.32 A typical polymerization procedure is described as follows. Acetic acid (30.025 g) was added dropwise into triethylamine (50.595 g) at 353 K. After stirring for 5 h, PIL which was yellow was obtained. PIL was characterized by 1H-NMR. 1H-NMR (CDCl3) δ: 7.979 (s, 1H, –NH), 2.762 (s, 3H, CH3COO–), 1.277–1.86 (t, 2H, –CH2−), 0.86 (s, 3H, –CH3), which is consistent with literature reports.2.3 Synthesis of HPS
Similarly, a series of hierarchical porous materials were synthesized by changing the temperature and the pH of the solution during synthesis. The final products were denoted as HAM-X-Y, in which X represented the aging temperature, Y represented the pH, respectively.
2.4 Catalysts preparation
1.0 g of hierarchical porous materials were inserted in a 20 mL ethanol solution containing 0.4 g of 12-tungstophosphoric acid at 333 K. The mixture continuously stirred until ethyl alcohol steamed. The impregnated white solid was dried at 393 K overnight and calcined in air at 573 K for 4 h.2.5 Catalytic tests
The alkylation reactions were carried out in a continuously stirred oil batch reactor under 393 K. Styrene (6.00 g), o-xylene (45.00 g) (quality ratio of o-xylene to styrene, 7.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and 1.02 g of catalyst were introduced in a three-neck 100 mL round-bottom flask equipped with a condenser for 3 h. Firstly, a small amount of o-xylene were added to the round-bottom flask at 393 K, followed by the desired amount of catalyst, the mixture of a certain amount of styrene and the remaining o-xylene then dropwise into the flask for 2 h. The final reaction mixture remained for another 1 h. After the reaction, unreacted o-xylene was distilled out under atmospheric pressure, and then the obtained liquid was denoted by crude product which was analyzed with GC-9890A gas chromatograph equipped with OV-1 capillary column and a flame ionization detector (FID). The yield of PXE was defined as follows:
1) and 1.02 g of catalyst were introduced in a three-neck 100 mL round-bottom flask equipped with a condenser for 3 h. Firstly, a small amount of o-xylene were added to the round-bottom flask at 393 K, followed by the desired amount of catalyst, the mixture of a certain amount of styrene and the remaining o-xylene then dropwise into the flask for 2 h. The final reaction mixture remained for another 1 h. After the reaction, unreacted o-xylene was distilled out under atmospheric pressure, and then the obtained liquid was denoted by crude product which was analyzed with GC-9890A gas chromatograph equipped with OV-1 capillary column and a flame ionization detector (FID). The yield of PXE was defined as follows:| Actual product weight = crude product weight × PXE (chromatography)% |
2.6 Characterization
The N2 physical adsorption and desorption isotherms were adopted at 77 K to obtain surface areas with ASAP 2020 apparatus (Micromertics USA) by means of the Brunauer–Emmett–Teller (BET) method. The pore size distribution in mesopore range was analyzed by the BJH (Barrett–Joyner–Halenda) method using the Halsey equation for multilayer thickness. Micropore volume was calculated by the t-plot method. Transmission Electron Microscopy (TEM) was performed on FEI Tecnai G2 T20 instrument. Scanning electron microscopy (SEM) images were recorded on a JEOL JSM-5600L SEM Instrument with a working distance of 3–4 mm and an electron voltage of 3.0 kV.3. Results and discussion
3.1 Effect of the hydrothermal temperature
Fig. 1A shows the N2 adsorption isotherms of the calcined samples at different hydrothermal temperature, and exhibits the pronounced hysteresis loop characterizing the mesopores. Including, the samples HAM-373 and HAM-393 possess an H1 type hysteresis loop attributed to the predominant mesoporous structure. In a sharp contrast, the adsorption of HAM-393 is higher than that of HAM-373, showing the order degree of HAM-393 is better than HAM-373. Whereas for HAM-353, the distortion of hysteresis loop illustrates the loss of the ordered pore structure. At very low relative pressure p/p0, an increase at the adsorption isothermal of HAM-373 and HAM-393 proves the presence of micropores. Fig. 1B shows the pore size distribution calculated by BJH model based on desorption curves. It can be seen that the diameter of the mesopore increases with the increase of hydrothermal temperature. There is one peak for HAM-353, which gives clear evidence that the low temperature has a bad influence on the behavior of templates, which is in agreement with the results of Table 1. And surface area and pore volume also have an increase when the temperature changes from 353 K to 393 K. On the basis of the above experimental results and the theory analysis, it is concluded that the hydrothermal temperature is a significant requirement to obtain the micro/mesoporous material. | ||
| Fig. 1 (A) N2 adsorption–desorption isotherms, (B) pore size distributions calculated by BJH model based on desorption curves of the calcined samples at different temperature when the pH was 2. | ||
| Sample name | BET surface area (cm2 g−1) | Pore volume (cm3 g−1) | Average pore size (nm) |
|---|---|---|---|
| UAM-393 | 788 | 0.67 | 3.40 |
| HAM-393 | 796 | 0.86 | 4.35 |
| HAM-373 | 703 | 0.81 | 4.60 |
| HAM-353 | 661 | 0.46 | 2.78 |
Fig. 2 shows the TEM images of HAM synthesized at different temperature. Two different kinds of mesoporous channels of all the materials are clearly observed. Furthermore, they are arranged in an ordered array, respectively. However, HAM-353 exhibits a little disordered structure which may be due to the low aging temperature. And all the samples show uniformly distributed pores and the pore size of the larger mesopore decreases to minor value when the temperature drops from 393 K to 353 K, which is in agreement with the result of N2 gas sorption. A part of the walls of mesoporous channels are destroyed a little in the enlarged picture, which may due to the existence of worm-like micropores.
3.2 Effect of the pH
The N2 adsorption isotherms of the calcined samples at different pH are shown in Fig. 3A. As previously described, the sample HAM-393-2 has a pronounced hysteresis loop, which shows that the structure remains the ordered channels. While, the order degree of the structure decreases with the increasing of pH. Moreover, the adsorption of the sample HAM-393-3 is larger than that of the sample HAM-393-2. It is worth noticing that a pronounced hysteresis loop at high relative pressure p/p0 of HAM-393-9 proves the presence of mesopores. Furthermore, it can be observed that the sample HAM-393-3 has a large pore diameter from Fig. 3B. In the contrast, the sample HAM-393-2 possesses a uniform and small pore diameter, showing that pH is an important factor for the material's structure. The characteristic data on the samples are summarized in Table 2. From Table 2, it is known that the surface area of the sample HAM-393-2 is much larger than the sample HAM-393-3 and the sample HAM-393-9, as high as 796 cm2 g−1. And the minor pore diameter may be the reason for the large surface area of the sample HAM-393-2. Moreover, it is shown that the sample HAM-393-3 possesses extremely large pore volume of 2.26 cm3 g−1, which are larger than that of HAM-393-2 of 0.86 cm3 g−1 and HAM-393-9 of 1.18 cm3 g−1. Furthermore, the pore size of HAM-393-3 and HAM-393-9 are above 20 nm. Therefore, it can be concluded that the different interaction between the P123 micelles and PIL micelles contributes to the final structure of the materials. | ||
| Fig. 3 (A) N2 adsorption–desorption isotherms, (B) pore size distributions calculated by BJH model based on desorption curves of the calcined samples induced by P123/PIL co-templates at 393 K. | ||
| Sample name | BET surface area (cm2 g−1) | t-plot micropore area (cm2 g−1) | Pore volume (cm3 g−1) | Average pore size (nm) |
|---|---|---|---|---|
| HAM-393-2 | 796 | 39 | 0.86 | 4.35 |
| HAM-393-3 | 368 | 43 | 2.26 | 24.59 |
| HAM-393-9 | 226 | 17 | 1.18 | 20.79 |
Fig. 4 shows the TEM images of HAM synthesized at different pH. Two different kinds of mesoporous channels of the sample HAM-393-2 are vividly observed. While, the sample HAM-393-3 possesses a structure like mesocellular foams and the sample HAM-393-9 exhibits a ball-like structure without regulation. This phenomenon may be related to the interaction between P123 and PIL. At strong acid condition, an ordered structure with two kinds of mesoporous channels which are induced by P123/PIL mixed micelles and PIL micelles are obtained. When the pH increases to a high value, the PIL micelles can enter the core of the P123 micelles. Finally, the mesocellular foams with large pore diameter are found. However, the disordered and ball-like material can be obtained because of the fact that PIL micelles and P123 micelles can't be fabricated under alkaline conditions. So, it is very important to control the reaction condition to get the desired structure.
 | ||
| Fig. 4 TEM images of the HAM at different pH. The insets are the local enlargements with the same enlargement scale. | ||
3.3 Effect of the synthesis route
The N2 adsorption–desorption isotherms of HAM and UAM are displayed in Fig. 5. Obviously, both of the adsorption isotherms exhibit the typical IV adsorption with a pronounced hysteresis loop which indicates the intersection network of mesoporous structures. At very low relative pressure p/p0, an increase at the adsorption isothermal of both materials proves the presence of micropores. Distortion of the characteristic hysteresis loop of UAM demonstrates the loss of ordered pore structure. It gives evidence that ultrasonic-assisted method may affect the behavior of the P123 micelles and the PIL micelles, resulting in the obtained HPS exhibits a disordered structure, which is consistent with the pore size distribution calculated by BJH model based on desorption curves. From Fig. 5B, it can be seen that the sample HAM possesses two peaks, indicating the material has two different mesopore ranges of pores of 4.0 nm and 7.3 nm, which induced by PIL micelles and P123 micelles, respectively. This phenomenon gives clear evidence that protic ionic liquid can self-assemble to micelles which act as the mesoporous templates at higher PIL concentration. Meanwhile, the sample UAM just has one peak, which may be caused by the fact that microwave may influence the formation of the ordered structure.The characteristic data of the samples are summarized in Table 1. From Table 1, It is shown that the total surface area of HAM-393 and UAM-393 are similar, about 790 cm2 g−1. However, the sample HAM possesses extremely large pore volume of 0.86 cm3 g−1, which are larger than that of the sample UAM of 0.67 cm3 g−1. In addition, the average pore size of HAM is 4.35 nm, which is larger than UAM as well. The existence of mixed micelles with large size is beneficial for the structure of HAM.
The TEM images of the calcined samples prepared at 393 K show the structure of the samples obtained with different synthesis method. The mesoporous channels with a number of disordered worm-like pores can be seen from the sample HAM. Moreover, there are two kinds of mesoporous channels which may be induced by the P123 micelles and PIL micelles. And the mesoporous channels are clearly found from the sample UAM. While, the order degree of the structure decreases, this is consistent with the N2 gas sorption results. This phenomenon may be caused by the fact that strong ultrasonic wave may discourage the form of P123 micelles and the self-assembly behavior between P123 and PIL (Fig. 6).
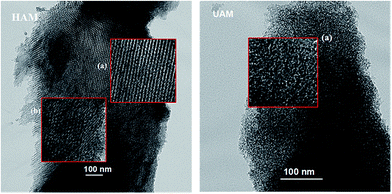 | ||
| Fig. 6 The images of the calcined samples induced by different methods when the pH was 2 at 393 K. The insets are the local enlargements with the same enlargement scale. | ||
The morphology of the samples prepared at 393 K is shown in the SEM image Fig. 7. It can be clearly seen that there is a difference between HAM-393-2 and HAM-393-3. Including, the sample HAM-393-2 possesses the large block structure, and the mesoporous channels can be obviously observed in the surface of the block. Meanwhile, it is worth noting that the sample HAM-393-3 shows the scattered schistose morphology. When the pH changes to 9, the structure without ordered morphology is found. The sample obtained by the ultrasonic-assisted method shows a special morphology with the ellipse structure, which is different from the sample HAM-393-2. The reason for this phenomenon may be the different interaction between P123 and PIL under different condition.
Formation mechanism of hierarchically porous silica materials: P123 molecules can self-assemble into the mesoscale micelles in aqueous systems, due to the fact that P123 have the hydrophobic core and the hydrophilic head group. In addition, ILs can also self-assemble into ordered structures like surfactants in an aqueous solution because of the special structure of IL which comprises both a hydrophilic ionic headgroup and a hydrophobic organic chain.20,33,34 The micelles with two different sizes are formed because of the different size of the PIL molecular and P123 molecular. It is known that there is a strong interaction between PIL and P123 mainly due to the hydrogen bond. Therefore, PIL micro scale micelles can surround the P123 mesoscale micelles in aqueous systems. As a result, the large micelles with two different sizes lead to the presence of two different kinds of mesopores at strong acid condition, which is certified by pore size distribution and TEM images of the samples synthesized at pH = 2 by hydrothermal method. It is said that the protic ionic liquid can act as multiple roles of cosolvent, cosurfactant as well as salt in the system. Under the favorable pH condition, PIL probably inclines to swell the P123 micelles. As a result, the increase of P123/PIL mixed aggregates' diameter is inevitable. In this case, the function of the PIL is analogous to the diameter expander, reagent 1,3,5-trimethylbenzene (TMB). In the example of P123/TMB mixed micelles, TMB molecules are totally solubilized in the core of P123 micelles, which obviously forms the swollen P123 micelles, finally, the size of mixed micelles can be higher than P123 micelles. Similarly, PIL also can enter the core of the P123 micelles, which lead to the partial miscibility in the core, consequently resulting in the size slightly increasing, which is confirmed by the N2 gas sorption and TEM results. The formation mechanism of the hierarchical porous silica material which is based on the interaction between PIL and P123 is schematically presented in Fig. 8.
3.4 Catalytic activity of materials supported HPW
The catalytic performance of different carriers with HPW were investigated due to the carriers possess high surface area and narrow size distribution which are advantageous to the reaction. And the catalytic activity of different catalysts is showed in Table 3. It is found that pure HPW shows high catalytic performance for the reaction, nevertheless, it is difficult to separate the HPW from the product mixture. Moreover, the HAM support itself shows no activity performances, which is consistent with literature reports.35 From Table 3, it is shown that both of the samples exhibit the higher catalytic properties than HPW/SBA-15. Among the catalysts investigated, HPW/HAM-393-3 with large pore size of the support exhibited the best catalytic performance. Combined with the data of Table 1, it can be seen that although the HAM-393-2 has a larger specific surface area, but its pore size is small, is not conducive to the macromolecular reaction. In contrast, the HPW/HAM-393-3 catalyst has a larger pore size, is beneficial to the reaction. Therefore, the catalytic activity may change along with the pore size and the surface area of supports. That is to say the large surface area with suitable pore size may contribution to the high catalytic performance.| Catalyst | Yieldb (%) | Selectivityc (%) |
|---|---|---|
a Reaction conditions: o-xylene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) styrene = 7.5 styrene = 7.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, reaction temperature = 393 K, reaction time = 3.0 h, catalyst loading = 20% (w/w of styrene).b Isolated yield based on the amount of styrene.c Target product: all products. 1, reaction temperature = 393 K, reaction time = 3.0 h, catalyst loading = 20% (w/w of styrene).b Isolated yield based on the amount of styrene.c Target product: all products. |
||
| HPW | 97.9 | 97.9 |
| HAM | — | — |
| HPW/SBA-15 | 68.2 | 75.1 |
| HPW/HAM-393-2 | 86.3 | 86.5 |
| HPW/HAM-393-3 | 95.5 | 95.8 |
4. Conclusion
In this work, a series of hierarchical porous silicas (HPS) have been successfully fabricated using P123 and protic ionic liquid in aqueous systems. The structure of the final materials was systematically investigated by three important factors: the pH of the solution, the hydrothermal temperature, the synthesis route. The results established that HPS with different structure were obtained under the different reaction condition. On the basis of the experimental results and the theory analysis, a possible formation mechanism of the hierarchical porous silica was illustrated. It was found that the hierarchical porous material with two kinds of mesoporous channels was fabricated under the strong acid condition. In this case, protic ionic liquid played a role of co-template. The mesocellular foams were formed when the pH increased to 3, while, silica spheres with small size were synthesized at pH = 9. This phenomenon may be explained by the fact that all kinds of structures with different morphologies and internal structures could be obtained through controlling the condition during the reaction. Moreover, the catalytic performances of the hierarchical porous materials supported HPW catalysts were investigated in the alkylation of o-xylene with styrene. Alkylation results showed that catalysts had good activity, which may be related to the large pore size and high surface area of the supports. Accordingly, it is concluded that the hierarchically porous silicas with special structure exhibited large pore volumes, diameter and may be useful in the fields of catalysis, adsorption, biological separation, and others.Acknowledgements
The authors are grateful to the financial supports of National Natural Science Foundation of China (Grant no. 21306023, 21376051, 21106017 and 51077013), Fund Project for Transformation of Scientific and Technological Achievements of Jiangsu Province of China (Grant no. BA2011086), Key Program for the Scientific Research Guiding Found of Basic Scientific Research Operation Expenditure of Southeast University (Grant no. 3207043101) and Instrumental Analysis Fund of Southeast University.References
- Y.-S. Ooi and S. Bhatia, Microporous Mesoporous Mater., 2007, 102, 310–317 CrossRef CAS PubMed.
- T. Welton, Chem. Rev., 1999, 99, 2071–2083 CrossRef CAS PubMed.
- J. H. Clark, D. J. Macquarrie and S. J. Tavener, Dalton Trans., 2006, 4297–4309 RSC.
- Y. H. Lin, Z. H. Li, Z. W. Chen, J. S. Ren and X. G. Qu, Biomaterials, 2013, 34, 2600–2610 CrossRef CAS PubMed.
- C. H. Christensen, K. Johannsen, I. Schmidt and C. H. Christensen, J. Am. Chem. Soc., 2003, 125, 13370–13371 CrossRef CAS PubMed.
- T. L. Chew, A. L. Ahmad and S. Bhatia, Adv. Compos. Mater., 2010, 153, 43–57 CAS.
- H. Zhang, A. Goeppert, M. Czaun, G. K. S. Prakash and G. A. Olah, RSC Adv., 2014, 4, 19403–19417 RSC.
- J. Q. Zhao, F. Simeon, Y. J. Wang, G. S. Luo and T. A. Hatton, RSC Adv., 2012, 2, 6509–6519 RSC.
- J. L. Vivero-Escoto, I. I. Slowing, B. G. Trewyn and V. S. Y. Lin, Small, 2010, 6, 1952–1967 CrossRef CAS PubMed.
- L. M. Xiong, J. L. Shi, L. X. Zhang and M. Y. Nogami, J. Am. Chem. Soc., 2007, 129, 11878–11879 CrossRef CAS PubMed.
- X. Chen, Z. Q. Sun, L. L. Zheng, Z. M. Chen, Y. F. Wang, N. Fu, K. Zhang, X. Yan, H. Liu, L. Jiang and B. Yang, Adv. Mater., 2004, 16, 1632–1636 CrossRef CAS PubMed.
- T. W. Kim, F. Kleitz, B. Paul and R. Ryoo, J. Am. Chem. Soc., 2005, 127, 7601–7610 CrossRef CAS PubMed.
- P. Schmidt-Winkel, W. W. Lukens, D. Y. Zhao, P. D. Yang, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc., 1999, 121, 254–255 CrossRef CAS.
- K. Egeblad, C. H. Christensen, M. Kustova and C. H. Christensen, Chem. Mater., 2008, 20, 946–960 CrossRef CAS.
- A. Noda, A. B. Susan, K. Kudo, S. Mitsushima, K. Hayamizu and M. Watanabe, J. Phys. Chem. B, 2003, 107, 4024–4033 CrossRef CAS.
- H. Li, P. S. Bhadury, B. A. Song and S. Yang, RSC Adv., 2012, 2, 12525–12551 RSC.
- C. C. Ke, J. Li, X. J. Li, Z. G. Shao and B. L. Yi, RSC Adv., 2012, 2, 8953–8956 RSC.
- Z. Ma, J. H. Yu and S. Dai, Adv. Mater., 2010, 22, 261–285 CrossRef CAS PubMed.
- P. Hapiot and C. Lagrost, Chem. Rev., 2008, 108, 2238–2264 CrossRef CAS PubMed.
- Y. Zhou and M. Antonietti, Adv. Mater., 2003, 15, 1452–1455 CrossRef CAS PubMed.
- Y. Zhou, J. H. Schattka and M. Antonietti, Nano Lett., 2004, 4, 477–481 CrossRef CAS.
- J. Hu, J. X. Yin, T. S. Lin and G. T. Li, J. Chem. Educ., 2012, 89, 284–285 CrossRef CAS.
- F. Gao, J. Hu, C. J. Peng, H. L. Liu and Y. Hu, Langmuir, 2012, 28, 2950–2959 CrossRef CAS PubMed.
- C. L. Tong, E. Eroglu and C. L. Raston, RSC Adv., 2014, 4, 46718–46722 RSC.
- S. Y. Chen, Y. T. Chen, J. J. Lee and S. Cheng, J. Mater. Chem., 2011, 21, 5693–5703 RSC.
- W. Wang, W. J. Shan, X. Y. Yue and H. Q. Ru, J. Mater. Chem., 2012, 22, 3462–3470 RSC.
- X. F. Zhou, A. J. Duan, Z. Zhao, Y. J. Gong, H. D. Wu, Y. C. Wei, G. Y. Jiang and J. Liu, Mater. Lett., 2014, 133, 228–231 CrossRef CAS PubMed.
- S. An, J. Joo and J. Lee, Microporous Mesoporous Mater., 2012, 151, 450–456 CrossRef CAS PubMed.
- A. Kumar and P. Venkatesu, RSC Adv., 2013, 3, 362–367 RSC.
- T. L. Greaves, A. Weerawardena, C. Fong, I. Krodkiewska and C. J. Drummond, J. Phys. Chem. B, 2006, 110, 22479–22487 CrossRef CAS PubMed.
- S. Tsuzuki, W. Shinoda, M. S. Miran, H. Kinoshita, T. Yasuda and M. Watanabe, J. Chem. Phys., 2013, 139, 174504 CrossRef PubMed.
- J. Y. Weng, C. M. Wang, H. R. Li and Y. Wang, Green Chem., 2006, 8, 96–99 RSC.
- D. B. Kuang, T. Brezesinski and B. Smarsly, J. Am. Chem. Soc., 2004, 126, 10534–10535 CrossRef CAS PubMed.
- Z. Du, E. Li, G. Wang and F. Cheng, RSC Adv., 2014, 4, 4836–4838 RSC.
- X. L. Sheng, J. Kong, Y. M. Zhou, Y. W. Zhang, Z. W. Zhang and S. J. Zhou, Microporous Mesoporous Mater., 2014, 187, 7–13 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |





