Synthesis of diverse α,ω-telechelic polystyrenes with di- and tri-functionality via tandem or one-pot strategies combining aminolysis of RAFT-polystyrene and a thiol–ene “click” reaction
Shuang-Shuang Zhangab,
Kun Cuib,
Jin Huangb,
Qiao-Ling Zhaob,
Shao-Kui Caoa and
Zhi Ma*b
aSchool of Materials and Engineering, Zhengzhou University, Zhengzhou, 450052, P. R. China
bKey Laboratory of Synthetic and Self-Assembly Chemistry for Organic Functional Molecules, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai, 200032, P. R. China. E-mail: mazhi728@sioc.ac.cn
First published on 8th May 2015
Abstract
A thiol–ene “click” reaction proceeds with facile reaction conditions for complete conversion, and displays a higher tolerance to various backbones and functional groups compared with traditional coupling and functionalization strategies. Herein, well-defined α,ω-telechelic polystyrenes with trithiocarbonate and carboxyl terminal groups (PS-CTA) were firstly synthesized via reversible addition-fragmentation chain transfer (RAFT) radical polymerization. Then, the terminal thiol group was converted from the thiocarbonylthio end group of PS-CTA and subsequently reacted with n-butyl acrylate and vinyl ferrocene, respectively, through thiol–ene “click” chemistry, to achieve α,ω-telechelic polystyrenes with difunctionality. Alternatively, a facile one-pot simultaneous aminolysis and thiol–ene “click” reaction using PS-CTA and various ene-bearing compounds as reactants was found to have high efficiency in synthesizing diverse α,ω-telechelic polystyrenes with di- and tri-functionality. Various functional groups such as hydroxyl, acrylate, fluorinated acrylate, ferrocene and allyl, etc. can be successfully incorporated as terminal groups of α,ω-telechelic polystyrenes.
Introduction
In the last 16 years, reversible addition-fragmentation chain transfer (RAFT) polymerization1 has increasingly been used to synthesize a wide range of well-defined polymers such as α-monofunctional polymers,2 α,ω-telechelic functional polymers3–12 and block copolymers.13–16 Polymers containing dithioester/thiocarbonylthio end groups synthesized by RAFT polymerization can be converted to thiol-terminated polymers by aminolysis with primary or secondary amines.8,17–21 Such thiol-terminated polymers can be further employed in the synthesis of functional materials such as polymers with a variety of terminal groups,21–25 surface modified transition metal nanoparticles or films,26–29 functional hairy hollow microspheres,30 temperature-responsive DNA-carrying polymer micelles,31 functionalized carbon nanotubes32 and well-defined polymers with diverse topological architectures,33–36 etc.More recently, thiol–ene chemistry has been introduced as a new “click” reaction and applied in many fields such as synthetic methodologies, biofunctionalization, surface modification, polymer and materials synthesis etc.37–43 The compounds or polymers bearing thiol group play an important role in thiol–ene “click” reaction and need to be synthesized with high thiol-functionality. However, the oxidative coupling of the thiol end groups observed during the aminolysis of dithioester/thiocarbonylthio end groups of polymers22,33,35,36,44–46 lead to bimodal polymer populations compose of thiol and disulfide polymers. In general, in order to avoid the oxidative coupling of thiols, the treatment of reaction mixture using Zn/acetic acid (Zn/HAc) after aminolysis47 and the addition of antioxidant sodium bisulfite in the system of animolysis4,5,48 was employed and found to be the efficient methodologies. In addition, NaBH4,26–31 the combination of amine and phosphine compounds,22,35,36,49 the combination of NaBH4 and phosphine compounds22 show their high capability for the reduction of dithioester/thiocarbonylthio end groups into thiols without the formation of disulfide byproduct. Phosphine compound was also utilized to cleave disulfide bond to generate thiol group at the chain end of block copolymer.50 Alternatively, a facile one-pot simultaneous aminolysis and thiol–ene “click” reaction7,11,35,36,51 using RAFT polymers and various thiol-reactive ene-bearing compounds as reactants showed high efficiency in synthesizing diverse α,ω-telechelic polymers without the formation of disulfide and the isolation of intermediate polymers.
Very recently, a variety of α-mono and α,ω-telechelic functional polystyrenes52–54 were synthesized via atomic transfer radical polymerization based methodology and employed for fabricating highly ordered honeycomb films.
In this work, new α,ω-telechelic polystyrenes with di- and tri-functionality bearing carboxyl, (fluorinated) alkyl ester, ferrocene and single/di-allyl ester were synthesized for the first time via tandem or one-pot strategies. In a tandem procedure, polystyrene with terminal thiocarbonylthio group (PS-CTA) by RAFT polymerization was firstly synthesized using S-1-dodecyl-S′-bis(α,α′-dimethyl-α′′-acetic acid)-trithiocarbonate (DDMAT)55 as chain transfer agent (CTA) and then transformed to α-thiol,ω-carboxyl telechelic polystyrene (HS–PS–COOH) via aminolysis with different reduction reagents. Subsequently, thiol–ene “click” reaction of HS–PS–COOH with n-butyl acrylate and vinylferrocene in the presence of photoinitiator was incandescent-lamp irradiated at room temperature to achieve α,ω-telechelic PS. Moreover, a facile one-pot strategy combining aminolysis of RAFT-polystyrene and thiol–ene “click” reaction simultaneously targeting diverse new α,ω-telechelic polystyrenes with di- and tri-functionality bearing carboxyl, (fluorinated) alkyl ester and single/di-allyl ester was reported.
Experimental
Materials
Styrene were dried over calcium hydride, distilled under reduced pressure, passed through a neutral alumina column to remove stabilizer, and degassed with nitrogen prior to use. The chain transfer agent of S-1-dodecyl-S′-(α,α′-dimethyl-α′′-acetic acid) trithiocarbonate (DDMAT) was synthesized as previously reported.55 n-Hexylamine (n-HA, Aladdin, 99%), acetic acid, tri-n-butylphosphine (Bu3P, Adamas, 98%+), 2,2-dimethoxy-2-phenylacetophenone (DMPA, Aladdin), NaBH3CN (J&K Scientific Ltd., 95%), n-butyl acrylate (n-BuA, Aladdin, 99%), allyl methacrylate (AMA) (Aldrich, 98%), 2,2,2-trifluoroethyl acrylate (TFEA) (J&K, 99%), 1,3,5-triallyl isocyanurate (TAIC, Aldrich, 98%) were used as received without further purification. Vinylferrocene (VCp2Fe) (>98%) was kindly provided by Prof. Jun Yang and Master candidate Mu-Shuang Qian in SIOC(CAS). Zinc powder (Aladdin) was purified by stirring in 3 wt% hydrochloric acid for 3 h, filtered and washed three times with deionized water and acetone. The purified zinc powder was then dried in vacuo and stored under dry nitrogen. Tetrahydrofuran (THF) were refluxed over sodium/benzophenone and distilled under N2 before use. 2,2-Azobis(isobutyronitrile) (AIBN) was purified by recrystallization from ethanol at 40 °C and dried under vacuum. Other reagents were purchased from Sinopharm Chemical Reagent, unless specified, and purified by standard procedures. All manipulations involving air- and/or moisture-sensitive compounds were carried out in a N2-filled dry box or using Schlenk techniques.Synthesis of α-thiol,ω-carboxyl telechelic PS (HS–PS–COOH)
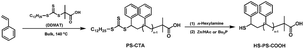
The synthetic procedure of α-thiol,ω-carboxyl telechelic PS (HS–PS–COOH) was depicted in Scheme 1. Firstly, in a similar procedure reported previously,56 styrene (16.2 mL, 141.5 mmol) and S-1-dodecyl-S′-(α,α′-dimethyl-α′′-acetic acid) trithiocarbonate (DDMAT) (1.45 g, 3.97 mmol) were added to a 50 mL Schlenk flask equipped with a stirring bar. The mixture was degassed with three freeze–evacuate–thaw cycles, followed by heating to 140 °C for 6 h in a thermostated oil bath. The flask was quenched to 0 °C, then the PS was isolated by precipitation of the reaction mixture into methanol, filtered and washed three times with methanol, and dried in a vacuum oven at room temperature for 24 h. PS-CTA: Mn = 3200 g mol−1, Mw/Mn = 1.08. 1H NMR (CDCl3): δ (ppm) = 7.30–6.30 (m, Hd), 5.03–4.60 (b, He), 3.34–3.19 (m, Hc), 2.50–1.13 (m, Hb+f+g+h+i2), 1.02–0.82 (m, Ha+i1). FT-IR (KBr), max 3082, 3059, 3025 (vs., C–H on phenyl); 2922 (s, –CH3); 2849 (m, –CH2–); 1743 (vs., C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1601, 1583, 1493, 1452 (s, C–C on phenyl); 1069 (s, C
O); 1601, 1583, 1493, 1452 (s, C–C on phenyl); 1069 (s, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S); 841 (s, –C–S); 756, 697 (as, –CH– on phenyl) cm−1.
S); 841 (s, –C–S); 756, 697 (as, –CH– on phenyl) cm−1.
Subsequently, PS-CTA (1.01 g, 0.32 mmol) was dissolved in 20 mL of anhydrous THF. The solution was purged with dry nitrogen for 15 min and a 10-fold molar excess n-hexylamine was added and then stirred for 1 h at room temperature. After that, the treatment of such reaction mixture was performed by adding Zn/HAc (molar ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) or tri-n-butylphosphine (Bu3P) (5 equiv. relative to thiol moiety) shown in Scheme 1. HS–PS–COOH was isolated by precipitation of the reaction mixture into methanol, filtered and washed three times with methanol, and dried in a vacuum oven at room temperature for 24 h. Mn(GPC) = 3076 g mol−1, Mw/Mn = 1.08. 1H NMR (CDCl3): δ (ppm) = 7.30–6.30 (m, Hd), 3.60–3.40 (s, He′), 2.50–1.32 (m, Hf+g+h), 1.32–1.10 (s, Hi2), 1.02–0.81 (m, Hi1).
1) or tri-n-butylphosphine (Bu3P) (5 equiv. relative to thiol moiety) shown in Scheme 1. HS–PS–COOH was isolated by precipitation of the reaction mixture into methanol, filtered and washed three times with methanol, and dried in a vacuum oven at room temperature for 24 h. Mn(GPC) = 3076 g mol−1, Mw/Mn = 1.08. 1H NMR (CDCl3): δ (ppm) = 7.30–6.30 (m, Hd), 3.60–3.40 (s, He′), 2.50–1.32 (m, Hf+g+h), 1.32–1.10 (s, Hi2), 1.02–0.81 (m, Hi1).
Synthesis of α,ω-telechelic PS via thiol–ene “click” reaction
In a similar procedure reported by Uygun and coworkers,57 a solution of HS–PS–COOH (1 equiv.), ene (n-BuA or VCp2Fe) (10 equiv.), photoinitiator (DMPA, 5 equiv.) in 10 mL of THF were introduced in a Schlenk flask and incandescent-lamp irradiated at room temperature (RT) for 2–7 h. The intensity of electric current was 25 A as measured by Xenon lamp XQ500W adjustable radiometer. The reaction mixture was precipitated in methanol and the obtained polymers were reprecipitated in acetonitrile, filtered and washed three times with acetonitrile, and then dried in a vacuum oven at room temperature for 24 h. The synthetic procedure was shown in Scheme 2. R1–S–PS–COOH (ene: n-BuA): Mn(GPC) = 3347 g mol−1, Mw/Mn = 1.13. Mn(MALDI-TOF) = 3335 g mol−1, Mw/Mn = 1.04. 1H NMR (CDCl3): δ (ppm) = 7.36–6.30 (m, Hd), 4.13–3.90 (b, Hl), 3.40–3.20 (s, He′′), 2.50–2.32 (s, Hk), 2.31–2.16 (s, Hj), 2.15–1.30 (m, Hf+g+h+m+n), 1.29–1.10 (s, Hi2), 1.02–0.79 (m, Hi1+p). R2–S–PS–COOH (ene: VCp2Fe): Mn(GPC) = 3602 g mol−1, Mw/Mn = 1.02. Mn(MALDI-TOF) = 3480 g mol−1, Mw/Mn = 1.02. 1H NMR (CDCl3): δ (ppm) = 7.36–6.28 (m, Hd), 4.50–3.60 (m, Hl), 3.59–3.40 (b, Hk), 3.25–3.06 (b, He′′), 2.34–2.16 (s, Hj), 1.23–1.16 (s, Hi2), 1.03–0.80 (m, Hi1). FT-IR (KBr): max 3082, 3059, 3025 (vs., C–H on phenyl); 2922 (s, –CH3); 2849 (m, –CH2–); 1743 (vs., C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1601, 1583, 1493, 1452 (s, C–C on phenyl); 1261.0, 1105.1, 807.0 (m, C–H on dicyclopentadienyl ring); 756, 697 (as, –CH– on phenyl) cm−1; 625 (w, –C–S–) cm−1. The content of Fe was 1.35 wt% by elemental analysis.
O); 1601, 1583, 1493, 1452 (s, C–C on phenyl); 1261.0, 1105.1, 807.0 (m, C–H on dicyclopentadienyl ring); 756, 697 (as, –CH– on phenyl) cm−1; 625 (w, –C–S–) cm−1. The content of Fe was 1.35 wt% by elemental analysis.
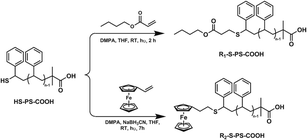 | ||
| Scheme 2 Synthesis of α,ω-telechelic polystyrenes via thiol–ene “click” reaction of between HS–PS–COOH and ene-bearing compound. | ||
Synthesis of α,ω-telechelic PS via one-pot strategy combining aminolysis of RAFT-polystyrene and thiol–ene “click” reaction simultaneously
In a typical synthetic procedure as shown in Scheme 3, PS-CTA (1 equiv., 0.01 mmol), ene (n-BuA, TFEA, AMA and TAIC, respectively) (10 equiv., 0.1 mmol) and DMPA (5 equiv., 0.05 mmol, only in the case of TFEA) were dissolved in THF (10 mL). n-Hexylamine (10 equiv., 0.1 m mol) was added dropwise under nitrogen. The solution was purged with nitrogen for 30 min. The solution was stirred in a Schlenk flask and irradiated at RT for 2–5 h. The intensity of electric current was 25 A as measured by Xenon lamp XQ500W adjustable radiometer. During the reaction, the characteristic yellow color of the solution disappeared. The product was purified in methanol (3 times) and dried in a vacuum oven at room temperature for 24 h. R3–S–PS–COOH (ene: TFEA): Mn(GPC) = 3432 g mol−1, Mw/Mn = 1.05. Mn(MALDI-TOF) = 3407 g mol−1, Mw/Mn = 1.10 1H NMR (CDCl3): δ(ppm) = 7.43–6.30 (m, Hd), 4.46–4.30 (m, Hl), 3.40–3.23 (m, He′′), 2.50–1.35 (m, Hf+g+h+k+j), 1.33–1.20 (s, Hi2), 1.06–0.82 (m, Hi1). 19F NMR (CDCl3): δ(ppm) = −73.67, −73.70, −73.72 ppm. R4–S–PS–COOH (ene: AMA): Mn(GPC) = 3674 g mol−1, Mw/Mn = 1.03. Mn(MALDI-TOF) = 3352 g mol−1, Mw/Mn = 1.02. 1H NMR (CDCl3): δ (ppm) = 7.30–6.30 (m, Hd), 5.94–5.70 (s, Hm), 5.35–5.11 (m, Hn), 4.60–4.35 (b, Hl), 3.40–3.20 (s, He′′), 2.50–1.29 (m, Hf+g+h+p+j), 1.29–1.15 (s, Hi2), 1.12–0.81 (m, Hk+i1). R5–S–PS–COOH (ene: TAIC): Mn(GPC) = 3308 g mol−1, Mw/Mn = 1.13. Mn(MALDI-TOF) = 3314 g mol−1, Mw/Mn = 1.02. 1H NMR (CDCl3): δ(ppm) = 7.30–6.30 (m, Hd), 5.96–5.70 (s, Hm), 5.41–5.10 (m, Hp), 4.55–4.34 (t, Hn), 3.87–3.60 (s, Hl), 3.42–3.30 (s, He′′), 3.22–2.97 (m, Hj), 2.54–1.20 (m, Hf+g+h+k), 1.18–1.07 (s, Hi2), 1.05–0.74 (m, Hi1).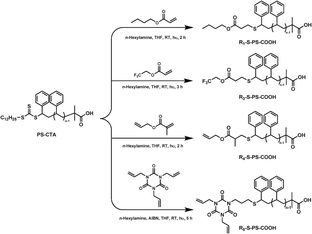 | ||
| Scheme 3 Synthesis of diverse α,ω-telechelic polystyrene via one-pot simultaneous aminolysis and thiol–ene “click” reaction of PS-CTA and ene-bearing compound. | ||
Polymers characterization
1H NMR and 19F NMR spectra of polymers were obtained on a Bruker AV 300 spectrometer (300 MHz) at room temperature with CDCl3 as the solvent. The number molecular weight (Mn) and molecular weight distribution (Mw/Mn) of polymers were measured by a Waters gel permeation chromatography (GPC) system equipped with a Waters 1515 Isocratic HPLC pump, a Waters 2414 refractive index detector (RI), a Waters 2487 dual k absorbance detector (UV) and a set of Waters Styragel columns (HR3, HR4 and HR5, 7.8 × 300 mm). GPC measurements were carried out at 35 °C using tetrahydrofuran (THF) as eluent with a flow rate of 4.0 μL min−1. The system was calibrated with polystyrene standards. FT-IR spectra were recorded on a Nicolet AVATAR-360 FT-IR spectrophotometer with a resolution of 4 cm−1. UV-vis spectra were recorded on U-3900 Spectrophotometer with scan speed of 2400 nm min−1 and sampling interval of 5.00 nm. Measurements were performed with a Shimadzu AXIMA Performance MALDI-TOF/TOFMS(matrix-assisted laser desorption and ionization time-of-flight) mass spectrometer, equipped with a nitrogen laser delivering 3 ns laser pulses at 337 nm. 1,8,9-Anthracenetriol (dithranol) was used as matrix. Samples were prepared by dissolving the polymer in dichloromethane at a concentration of 3 g L−1. A 1 μL aliquot of this solution was added to 10 μL of a 20 g L−1 matrix solution and 1 μL of a silver(I) trifluoroacetate solution (cationization agent). A 1 μL aliquot of the resulting mixture was applied to a multistage target to evaporate the dichloromethane and create a thin matrix/analyte film. The ions were measured in the reflectron mode of the spectrometer. Only lithium-cationized ions (M + Li+) were detected. TOFmix was used for an external calibration immediately before the measurement.Results and discussion
Synthesis of α-thiol,ω-carboxyl telechelic PS (HS–PS–COOH)
The synthetic procedure of α-thiol,ω-carboxyl telechelic PS (HS–PS–COOH) was illustrated in Scheme 1. Firstly, a well-defined thiocarbonylthio terminated polystyrene (PS-CTA) was obtained by bulk RAFT polymerization of styrene using DDMAT as chain transfer agent. Subsequently, PS-CTA was aminolysized using n-hexylamine and then treated by Zn/HAc or tri-n-butylphosphine (Bu3P).PS-CTA with molecular weight of Mn = 3200 g mol−1 (the molecular weight at peak Mp = 3509 g mol−1) was obtained and showed a single narrow peak (Mw/Mn = 1.08) in its GPC curve (Fig. 1(a)). 1H NMR spectrum of PS-CTA (Fig. 2(b)) confirmed its chain structure as reported previously.56 Upon cleavage of the thiocarbonylthio group at the chain end of PS-CTA, primary or secondary amines was well-known to react with thiocarbonylthio group rapidly at ambient temperature leading irreversibly to thioamides and thiols.4–8,17–21 Herein, PS-CTA was transformed into α-thiol,ω-carboxyl telechelic PS by aminolysis using n-hexylamine alone. The aminolysis of the thiocarbonylthio end group was confirmed by a rapid color change from yellow to colourless. However, the oxidative coupling of the thiol end-groups to produce disulfide was also observed by the presence of bimodal molecular weight distribution in GPC curve of the resulted polymer as shown in Fig. 1(b). The higher molecular weight at peak (Mp = 6537 g mol−1) was approximately twice as the smaller Mp (3435 g mol−1) which was a little lower than Mp = 3509 g mol−1 of PS-CTA (Fig. 1(a)) due to the transformation of thiocarbonylthio moiety into thiol moiety. It was attributed to the formation of disulfide (HOOC–PS–S–S–PS–COOH) by the oxidative coupling of HS–PS–COOH.
Despite taking all precautions, such as degassing the reaction mixture, the oxidative coupling of the thiol moiety can still occur upon treatment with n-hexylamine. To avoid the oxidative coupling of the thiol moiety, such reaction mixture after aminolysis was treated by Zn/HAc. A unimodal molecular weight distribution was observed in GPC curve (Fig. 1(c)) and its Mp (3396 g mol−1) was a little lower than Mp = 3509 g mol−1 of PS-CTA (Fig. 1(a)) because of the removal of thiocarbonylthio moiety from PS-CTA. As shown in 1H NMR spectra (Fig. 2), the singlet at 3.39–3.16 ppm assigned to the methylene proton (c) next to thiocarbonylthio (Fig. 2(a)) of PS-CTA could not be observed (Fig. 2(b)) after its aminolysis by n-hexylamine and the treatment of Zn/HAc. Meanwhile, the singlet at 5.03–4.60 ppm assigned to the methine proton (e) (–SC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)S–CH(Ph)–) of PS-CTA (Fig. 2(a)) shifted to 3.65–3.40 ppm, indicating the presence of methine proton (e′) (HS–CH(Ph)–). Moreover, the strong absorbance of thiocarbonylthio moiety of PS-CTA at 315 nm in UV-Vis spectra (Fig. 3) was almost completely disappeared also indicating the formation of HS–PS–COOH.
S)S–CH(Ph)–) of PS-CTA (Fig. 2(a)) shifted to 3.65–3.40 ppm, indicating the presence of methine proton (e′) (HS–CH(Ph)–). Moreover, the strong absorbance of thiocarbonylthio moiety of PS-CTA at 315 nm in UV-Vis spectra (Fig. 3) was almost completely disappeared also indicating the formation of HS–PS–COOH.
Alternatively, in order to avoid the oxidative coupling of thiol moiety, Na2S2O4 and Bu3P were employed as reducing agent to treat the reaction mixture of aminolysis, respectively. Neither of them showed advantage over Zn/HAc in our case.
Synthesis of α,ω-telechelic PS via thiol–ene “click” reaction
The thiol–ene “click” reactions between HS–PS–COOH and enes (n-butyl acrylate (n-BuA) and vinylferrocene (VCp2Fe), respectively) were carried out at room temperature (RT) by irradiation with an incandescent-lamp using DMPA as photoinitiator (see Scheme 2). The GPC and MALDI-TOF analysis of R1–S–PS–COOH showed similar molecular weights (Mn(GPC) = 3347 g mol−1 vs. Mn(MALDI-TOF) = 3335 g mol−1) and narrow molecular weight distributions (Mw/Mn = 1.13 and 1.04, respectively). The structure of polymer was ascertained by analysis of its 1H NMR spectrum. As shown in Fig. 4, the signal of the methine proton next to thiol moiety at 3.65–3.40 ppm (e′ in Fig. 2(b)) shifted to 3.40–3.20 ppm (e′′ in Fig. 4). Simultaneously, new singlets l (δ = 4.13–3.90 ppm), k (δ = 2.50–2.32 ppm) and j (δ = 2.31–2.16 ppm) were observed and assigned to protons of –CH2–O–CO–, –O–CO–CH2– and –CH2–S–, respectively. According to the analysis described above, the connection of thiol moiety with n-BuA via thiol–ene “click” reaction was confirmed and the targeting polymer R1–S–PS–COOH was achieved. | ||
| Fig. 4 1H NMR spectrum of R1–S–PS–COOH synthesized via tandem strategy combining aminolysis of PS-CTA and thiol–ene “click” reaction. | ||
Unexpectedly, in the same reaction condition, HS–PS–COOH didn't react with VCp2Fe. It was worth noting that the addition of NaBH3CN,58 a reducing agent with milder reactivity and easier manipulation than those of NaBH4, accelerated the thiol–ene “click” reaction between HS–PS–COOH and VCp2Fe. The possible explanation for such phenomenon is underwork.
As shown in Fig. 5, the signal of the methine proton next to thiol moiety at 3.65–3.40 ppm (e′, in Fig. 2(b)) shifted to 3.26–3.00 ppm (e′′ in Fig. 5). Simultaneously, new singlets l (δ = 4.50–3.60 ppm), k (δ = 3.59–3.40 ppm) and j (δ = 2.34–2.16 ppm), were present and assigned to protons of dicyclopentadienyl ring, Cp–CH2– and –CH2–S–, respectively. In addition, the characteristic infrared absorbances of C–H of dicyclopentadienyl ring at 1261.0, 1105.1, 807.0 cm−1 were appear. The molecular weight of R2–S–PS–COOH determined by GPC is 3602 g mol−1 (Mw/Mn = 1.02) and close to 3480 g mol−1 (Mw/Mn = 1.02) determined by MALDI-TOF. All the analysis described above indicated the formation of the targeting polymer R2–S–PS–COOH.
 | ||
| Fig. 5 1H NMR spectrum of R2–S–PS–COOH synthesized via tandem strategy combining aminolysis of PS-CTA and thiol–ene “click” reaction. | ||
Synthesis of α,ω-telechelic PS via one-pot strategy combining aminolysis of RAFT-polystyrene and thiol–ene “click” reaction simultaneously
As described above, the synthesis of thiol-terminated polymer (HS–PS–COOH) without any disulfide byproduct was the key to such tandem strategy targeting α,ω-telechelic PS. Thus, after the aminolysis of PS-CTA, an additional treatment of such reaction mixture by Zn/HAc had to be performed in order to avoid the formation of disulfide. Finally, the α,ω-telechelic PS was obtained by thiol–ene “click” reaction of HS–PS–COOH with ene-bearing compound. Alternatively, a facile one-pot strategy combining aminolysis with thiol–ene “click” reaction simultaneously7,11,35,36,51 showed its high efficiency and versatility in the synthesis of diverse α,ω-telechelic polymers avoiding the formation of disulfide-containing polymer byproduct and the isolation of intermediate polymers. Herein, we used such strategy to prepare four new α,ω-telechelic polystyrenes with di- and tri-functionality bearing carboxyl, alkyl ester, fluorinated alkyl ester and single/di-allyl ester under incandescent-lamp irradiation without photoinitiator as shown in Scheme 3.Firstly, for the comparison between one-pot and tandem strategies, n-BuA was employed as ene-bearing compound in the simultaneous aminolysis and thiol–ene “click” reaction irradiated by an incandescent-lamp without any photoinitiator at RT for 2 h. The analysis of the resulted polymer by 1H NMR (the same spectrum as that shown in Fig. 4) and UV-vis spectra (no absorbance of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S band at ∼315 nm) indicated the successful synthesis of R1–S–PS–COOH via such one-pot strategy with high efficiency. Subsequently, VCp2Fe was used to prepare R2–S–PS–COOH under a similar reaction condition but didn't work. The further research on this reaction system is underway.
S band at ∼315 nm) indicated the successful synthesis of R1–S–PS–COOH via such one-pot strategy with high efficiency. Subsequently, VCp2Fe was used to prepare R2–S–PS–COOH under a similar reaction condition but didn't work. The further research on this reaction system is underway.
One α-fluorinated alkyl ester,ω-carboxyl-telechelic PS (R3–S–PS–COOH) can be prepared in one-pot procedure using TFEA as ene-bearing compound under photoinitiation only in the presence of DMPA. The detail research was underway. The chain structure of the purified R3–S–PS–COOH was confirmed by UV-vis, 1H NMR and 19F NMR spectra. The disappearance of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S band at ∼315 nm in UV-vis spectrum indicated the removal of thiocarbonylthio moiety. As shown in Fig. 6, both of the singlets at 5.03–4.60 and 3.25 ppm assigned to the methine proton (e) (–SC(
S band at ∼315 nm in UV-vis spectrum indicated the removal of thiocarbonylthio moiety. As shown in Fig. 6, both of the singlets at 5.03–4.60 and 3.25 ppm assigned to the methine proton (e) (–SC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)S–CH(Ph)–) and methylene protons (c) next to thiocarbonylthio of PS-CTA (Fig. 2(a)) were disappeared, while new singlets at 4.40 and 3.30 ppm assigned to methylene protons (l) next to CF3 moitey and methine proton (e′′) (–CH2–S–CH(Ph)–) present. The incorporation of CF3 moiety was also confirmed by three singlets at −73.67, –73.70 and −73.72 ppm in 19F NMR spectrum. GPC and MALDI-TOF measurements showed similar molecular weight of R3–S–PS–COOH (Mn(GPC) = 3432 g mol−1 vs. Mn(MALDI-TOF) = 3407 g mol−1) with narrow molecular weight distribution (Mw/Mn = 1.10).
S)S–CH(Ph)–) and methylene protons (c) next to thiocarbonylthio of PS-CTA (Fig. 2(a)) were disappeared, while new singlets at 4.40 and 3.30 ppm assigned to methylene protons (l) next to CF3 moitey and methine proton (e′′) (–CH2–S–CH(Ph)–) present. The incorporation of CF3 moiety was also confirmed by three singlets at −73.67, –73.70 and −73.72 ppm in 19F NMR spectrum. GPC and MALDI-TOF measurements showed similar molecular weight of R3–S–PS–COOH (Mn(GPC) = 3432 g mol−1 vs. Mn(MALDI-TOF) = 3407 g mol−1) with narrow molecular weight distribution (Mw/Mn = 1.10).
 | ||
| Fig. 6 1H NMR spectrum of R3–S–PS–COOH synthesized via one-pot strategy combining aminolysis of PS-CTA and thiol–ene “click” reaction simultaneously. | ||
An α-allyl,ω-carboxyl-telechelic PS (R4–S–PS–COOH) was prepared efficiently via one-pot strategy using AMA as ene-bearing compound under photoinitiation without DMPA. Its chain structure was confirmed by the analysis of UV-vis and 1H NMR spectra (Fig.7). The GPC data (Mn(GPC) = 3674 g mol−1, Mw/Mn = 1.03) of R4–S–PS–COOH is close to that determined by MALDI-TOF(Mn(MALDI-TOF) = 3352 g mol−1, Mw/Mn = 1.02). Both the allyl and carboxyl moieties can be further employed to constructing new polymer architecture with various components and functions.
 | ||
| Fig. 7 1H NMR spectrum of R4–S–PS–COOH synthesized via one-pot strategy combining aminolysis of PS-CTA and thiol–ene “click” reaction simultaneously. | ||
Moreover, α,ω-telechelic PS with tri-functionality (R5–S–PS–COOH) was also synthesized using TAIC containing tri-allyl moieties to test the versatility of one-pot strategy. However, such reaction didn't go well without or with DMPA in an increasing reaction time of 5 h. After several trials, it was found that AIBN can accelerate the one-pot simultaneous aminolysis and thiol–ene “click” reaction between PS-CTA and TAIC forming R5–S–PS–COOH. The molecular weight (Mn(GPC) = 3308 g mol−1, Mw/Mn = 1.13) of R5–S–PS–COOH measured by GPC is almost the same as that determined by MALDI-TOF (Mn(MALDI-TOF) = 3314 g mol−1, Mw/Mn = 1.02). The characteristic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S band of PS-CTA at ∼315 nm disappeared completely. In the 1H NMR spectrum (Fig. 8) of the purified polymer, the appearance of new singlets of e′′ (δ = 3.36 ppm), j (δ = 3.10 ppm), l (δ = 3.70 ppm), m (δ = 5.85 ppm), p (δ = 5.25 ppm) and n (δ = 4.45 ppm) indicating the formation of R5–S–PS–COOH. The further study on the role of AIBN in such one-pot procedure and the application of R5–S–PS–COOH with di-allyl moieties is under investigation.
S band of PS-CTA at ∼315 nm disappeared completely. In the 1H NMR spectrum (Fig. 8) of the purified polymer, the appearance of new singlets of e′′ (δ = 3.36 ppm), j (δ = 3.10 ppm), l (δ = 3.70 ppm), m (δ = 5.85 ppm), p (δ = 5.25 ppm) and n (δ = 4.45 ppm) indicating the formation of R5–S–PS–COOH. The further study on the role of AIBN in such one-pot procedure and the application of R5–S–PS–COOH with di-allyl moieties is under investigation.
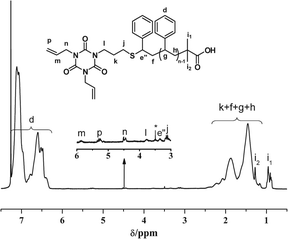 | ||
| Fig. 8 1H NMR spectrum of R5–S–PS–COOH synthesized via one-pot strategy combining aminolysis of PS-CTA and thiol–ene “click” reaction simultaneously. | ||
Conclusions
Diverse well-defined α,ω-functional telechelic polystyrenes with di- and tri-functionality were synthesized via tandem or one-pot strategies combining aminolysis of RAFT-polystyrene and thiol–ene “click” reaction under incandescent-lamp irradiation with even without photoinitiator. On the one hand, disulfide byproduct produced by the oxidative coupling of thiol moiety during the aminolysis of RAFT-polystyrene can be avoided efficiently by using such one-pot strategy. On the other hand, a variety of terminal functionalized polystyrenes bearing different moieties such as carboxyl, (fluorinated) alkyl ester, ferrocene and single/di-allyl ester were prepared for the first time and possibly find their applications in constructing polymers or their composites with new architecture and functionality.Acknowledgements
The authors greatly appreciate the financial support from the National Natural Science Foundation of China (nos 21074146, 21374130).Notes and references
- J. Chiefari, Y. K. Chong, F. Ercole, J. Krstina, J. Jeffery, T. P. T. Le, R. T. A. Mayadunne, G. F. Meijs, C. L. Moad and G. Moad, Macromolecules, 1998, 31, 5559–5562 CrossRef CAS.
- G. Moad, E. Rizzardo and S. H. Thang, Polymer, 2008, 49, 1079–1131 CrossRef CAS.
- J. Liu, C. Y. Hong and C. Y. Pan, Polymer, 2004, 45, 4413–4421 CrossRef CAS.
- D. L. Patton, M. Mullings, T. Fulghum and R. C. Advincula, Macromolecules, 2005, 38, 8597–8602 CrossRef CAS.
- V. Lima, X. L. Jiang, J. Brokken-Zijp, P. J. Schoenmakers, B. Klumperman and R. Van Der Linde, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 959–973 CrossRef CAS.
- G. Moad, Y. K. Chong, A. Postma, E. Rizzardo and S. H. Thang, Polymer, 2005, 46, 8458–8468 CrossRef CAS.
- X. P. Qiu and F. M. Winnik, Macromol. Rapid Commun., 2006, 27, 1648–1653 CrossRef CAS.
- F. Segui, X. P. Qiu and F. M. Winnik, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 314–316 CrossRef CAS.
- C. Boyer, J. Liu, V. Bulmus, T. P. Davis, C. Barner-Kowollik and M. H. Stenzel, Macromolecules, 2008, 41, 5641–5650 CrossRef CAS.
- M. J. Stanford and A. P. Dove, Macromolecules, 2009, 42, 141–147 CrossRef CAS.
- C. Boyer, A. Granville, T. P. Davis and V. Bulmus, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 3773–3794 CrossRef CAS.
- N. A. Cortez-lemus, R. Salgado-rodriguez and A. Licea-claverie, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 3033–3051 CrossRef CAS.
- A. B. Lowe and C. L. McCormick, Prog. Polym. Sci., 2007, 32, 283–351 CrossRef CAS.
- A. W. York, S. E. Kirkland and C. L. McCormick, Adv. Drug Delivery Rev., 2008, 60, 1018–1036 CrossRef CAS PubMed.
- J. T. Sun, C. Y. Hong and C. Y. Pan, Polym. Chem., 2013, 4, 873–991 RSC.
- D. J. Keddie, Chem. Soc. Rev., 2014, 43, 496–505 RSC.
- M. Delere and G. Levesque, Macromolecules, 1990, 23, 4733–4740 CrossRef.
- G. Levesque, P. Arsene, V. Fanneau-Bellenger and T. N. Pham, Biomacromolecules, 2000, 1, 400–406 CrossRef CAS PubMed.
- Y. Inoue, T. Matsugi, N. Kashiwa and K. Matyjaszewski, Macromolecules, 2004, 37, 3651–3658 CrossRef CAS.
- B. J. Kim, J. Bang, C. J. Hawker, J. J. Chiu, D. J. Pine, S. G. Jang, S. M. Yang and E. J. Kramer, Langmuir, 2007, 23, 12693–12703 CrossRef CAS PubMed.
- L. Feng, K. A. Cavicchi, B. C. Katzenmeyer and C. Wesdemiotis, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 5100–5108 CrossRef CAS.
- C. W. Scales, A. J. Convertine and C. L. McCormick, Biomacromolecules, 2006, 7, 1389–1392 CrossRef CAS PubMed.
- Y. B. Choi and O. O. Park, J. Appl. Polym. Sci., 2008, 109, 736–748 CrossRef CAS.
- G. Fei, J. Shin, S. A. Kang, B. S. Ko, P. H. Kang, Y. S. Lee and Y. C. Nho, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 563–569 CrossRef CAS.
- P. J. Roth, C. Boyer, A. B. Lowe and T. P Davis, Macromol. Rapid Commun., 2011, 32, 1123–1143 CrossRef CAS PubMed.
- A. B. Lowe, B. S. Sumerlin, M. S. Donovan and C. L. McCormick, J. Am. Chem. Soc., 2002, 124, 11562–11563 CrossRef CAS PubMed.
- B. S. Sumerlin, A. B. Lowe, P. A. Stroud, P. Zhang, M. W. Urban and C. L. McCormick, Langmuir, 2003, 19, 5559–5562 CrossRef CAS.
- J. W. Hotchkiss, A. B. Lowe and S. G. Boyes, Chem. Mater., 2007, 19, 6–13 CrossRef CAS.
- J. Li, W.-D. He and X.-L. Sun, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 5156–5163 CrossRef CAS.
- G. L. Li, L. Q. Xu, X. Tang, K. G. Neoh and E. T. Kang, Macromolecules, 2010, 43, 5797–5803 CrossRef CAS.
- K. Isoda, N. Kanayama, D. Miyamoto, T. Takarada and M. Maeda, React. Funct. Polym., 2011, 71, 367–371 CrossRef CAS.
- Y. Z. You, C. Y. Hong and C. Y. Pan, Macromol. Rapid Commun., 2006, 27, 2001–2006 CrossRef CAS.
- M. R. Whittaker, Y. K. Goh, H. Gemici, T. M. Legge, S. Perrier and M. J. Monteiro, Macromolecules, 2006, 39, 9028–9034 CrossRef CAS.
- H. Gemici, T. M. Legge, M. Whittaker, M. J. Monteiro and S. Perrier, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 2334–2340 CrossRef CAS.
- J. W. Chan, B. Yu, C. E. Hoyle and A. B. Lowe, Chem. Commun., 2008, 40, 4959–4961 RSC.
- J. W. Chan, B. Yu, C. E. Hoyle and A. B. Lowe, Polymer, 2009, 50, 3158–3168 CrossRef CAS.
- C. E. Hoyle, T. Y. Lee and T. Roper, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 5301–5338 CrossRef CAS.
- A. Dondoni, Angew. Chem., Int. Ed., 2008, 47, 8995–8997 CrossRef CAS PubMed.
- C. E. Hoyle, A. B. Lowe and C. N. Bowman, Chem. Soc. Rev., 2010, 39, 1355–1387 RSC.
- C. E. Hoyle and C. N. Bowman, Angew. Chem., Int. Ed., 2010, 49, 1540–1573 CrossRef CAS PubMed.
- A. B. Lowe, Polym. Chem., 2010, 1, 17–36 RSC.
- M. J. Kade, D. J. Burke and C. J. Hawker, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 743–750 CrossRef CAS.
- A. B. Lowe, Polym. Chem., 2014, 5, 4820–4870 RSC.
- J. Xu, J. He, D. Fan, X. Wang and Y. Yang, Macromolecules, 2006, 39, 8616–8624 CrossRef CAS.
- X. P. Qiu and F. M. Winnik, Macromolecules, 2007, 40, 872–878 CrossRef CAS.
- S. Harrisson, Macromolecules, 2009, 42, 897–898 CrossRef CAS.
- Z. Wang, J. He, Y. Tao, L. Yang, H. Jiang and Y. L. Yang, Macromolecules, 2003, 36, 7446–7452 CrossRef CAS.
- Y. Z. You and D. Oupicky, Biomacromolecules, 2007, 8, 98–105 CrossRef CAS PubMed.
- M. Li, P. De, S. R. Gondi and B. S. Sumerlin, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 5093–5100 CrossRef CAS.
- N. C. Kalarickal, S. Rimmer, P. Sarker and J.-C. Leroux, Macromolecules, 2007, 40, 1874–1880 CrossRef CAS.
- J. M. Spruell, B. A. Levy, A. Sutherland, W. R. Dichtel, J. Y. Cheng, J. F. Stoddart and A. Nelson, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 346–356 CrossRef CAS.
- L.-W. Zhu, Y. Ou, L.-S. Wan and Z.-K. Xu, J. Phys. Chem. B, 2014, 118, 845–854 CrossRef CAS PubMed.
- L.-W. Zhu, W. Yang, Y. Ou, L.-S. Wan and Z.-K. Xu, Polym. Chem., 2014, 5, 3666–3672 RSC.
- L.-W. Zhu, B.-H. Wu, L.-S. Wan and Z.-K. Xu, Polym. Chem., 2014, 5, 4311–4320 RSC.
- J. T. Lai, D. Filla and R. Shea, Macromolecules, 2002, 35, 6754–6756 CrossRef CAS.
- J.-P. Gao, W. Wu, L. Rong, G.-L. Mao, Y.-N. Ning, Q.-L. Zhao, J. Huang and Z. Ma, Eur. Polym. J., 2014, 59, 171–179 CrossRef CAS.
- M. Uygun, M. A. Tasdelen and Y. Yagci, Macromol. Chem. Phys., 2010, 211, 103–110 CrossRef CAS.
- C. F. Lane, Synthesis, 1975, 3, 135–146 CrossRef.
| This journal is © The Royal Society of Chemistry 2015 |