Lead biotransformation potential of allochthonous Bacillus sp. SKK11 with sesame oil cake extract in mine soil
Muthusamy
Govarthanan†
a,
Sung-Hee
Park†
b,
Yool-Jin
Park
c,
Hyun
Myung
c,
R. R.
Krishnamurthy
d,
Sang-Hyun
Lee
e,
Nanh
Lovanh
f,
Seralathan
Kamala-Kannan
*a and
Byung-Taek
Oh
*a
aDivision of Biotechnology, Advanced Institute of Environment and Bioscience, College of Environmental and Bioresource Sciences, Chonbuk National University, Iksan 570 752, South Korea. E-mail: kannan@jbnu.ac.kr; btoh@jbnu.ac.kr; Fax: +82-63-850-0834; Tel: +82-63-850-0842/+82-63-850-0838
bDepartment of Rehabilitation Medicine, School of Medicine, Chonbuk National University, Jeonju, Jeonbuk 561-756, South Korea
cDepartment of Ecology Landscape Architecture – Design, College of Environmental and Bioresource Sciences, Chonbuk National University, Iksan 570 752, South Korea
dDepartment of Applied Geology, School of Earth and Atmospheric Sciences, University of Madras, Chennai 600 025, India
eDepartment of Forest Environment Science, College of Agriculture & Life Sciences, Chonbuk National University, Jeonju 561-756, South Korea
fUSDA-ARS, AWMRU, 230 Bennett Lane, Bowling Green, KY 42104, USA
First published on 15th June 2015
Abstract
The potential of allochthonous Bacillus sp. SKK11 and sesame oil cake extract for the immobilization of Pb in mine soil was investigated in this study. The isolate SKK11, isolated from a brackish environment and identified as Bacillus sp. based on partial 16S rDNA sequencing, exhibited maximum resistance to Pb (750 mg L−1). Growth kinetic studies revealed that the presence of oil cake extract (2%) increased the biomass of the isolate SKK11. Transmission electron microscopy and X-ray diffraction studies showed that isolate SKK11 transformed Pb either intracellularly or extracellularly. Selective sequential extraction studies showed that the bioremediation decreased 24.9% of the exchangeable fraction in the mine soil in 3 days. However, 75.1% of the exchangeable fraction was not immobilized in the soil. An X-ray diffractogram of the bioremediated soil showed a major decrease (79.0%) in the intensity of the plagioclase mineral peak. Urease, dehydrogenase, amylase, invertase, cellulase, and alkaline phosphatase enzyme activities were increased in the bioremediated mine soil. These results suggest that the isolate Bacillus sp. SKK11 in combination with sesame oil cake extract could be employed for the immobilization of bioavailable Pb in contaminated soil.
1. Introduction
Heavy metal contamination poses a serious threat to both the environment and human health. Rapid industrialization, mining activities, disposal of metal wastes, usage of pesticides, and spillage of petrochemicals are the major sources of heavy metal pollution in the ecosystem.1 Elimination of heavy metals from the contaminated soil is particularly challenging as these metals are non-biodegradable. Among the heavy metals, lead (Pb) has been recognized as one of the most hazardous pollutants in the environment. Moreover, Pb is not an essential nutrient in the metabolic processes of plants and/or animals, and it can accumulate to high levels and become toxic to organisms.2,3 Thus, the development of remediation strategies for Pb polluted soils is important for ecological conservation and human health. Several chemical methods have been developed to control the dispersion and biomagnification of metals from contaminated soil.4 However, the disadvantages and ineffectiveness of chemical methods have been widely reported.5,6Biotransformation is an efficient selective bioremediation technology utilizing the potentiality of heavy metal resistant microorganisms to transform metal ions. A number of micro-organisms inhabiting soil and water can transform the active fraction of metals into inactive fractions, which diminishes the bioavailability and biomagnification of metals in food chain.7 Several studies reported that the bacterial strains such as Pseudomonas sp., Bacillus sp., Acinetobacter sp., Flavobacterium sp., Aeromonas sp.8–10 were capable of converting organic/inorganic forms of Pb into less toxic derivatives. However, survival of the bacteria in the contaminated soil is essential for biotransformation of Pb since these reactions are enzyme mediated.11
Bioaugmentation is the application of indigenous or allochthonous, wild type or genetically modified microorganisms to accelerate the removal of pollutants from contaminated sites.12 Recently, several groups of pollutants were successfully remediated/transformed using bioaugmentation. The arsenic tolerant bacterium Sporosarcina ginsengisoli significantly transformed the exchangeable fraction of arsenic in artificially contaminated soil.13 Similarly, bioaugmentation with siderophore producing bacteria significantly increased the phytoextraction rate of chromium (Cr) and lead (Pb).14 Yet the bacteria-based biotransformation of metals in mine soil is not so effective because the mine soil is regularly lacking in organic nutrients and cannot support bacterial growth. In addition, geological conditions, nutrient accessibility, and oxygen availability may limit the bacterial activity and biotransformation of metals.15
Mining activities alter the geochemical nature of the soil in a manner that prevents the rapid growth of bacteria.16 An approach to accelerate the metabolism and proliferation of microorganisms is the addition of nutrients to the contaminated matrix, i.e., biostimulation.17 The combined technology of bioaugmentation assisted by biostimulation integrates the effectiveness of both technologies and proposes a promising approach to the bioremediation of heavy metals.18 Hence, it is important to find an inexpensive and effective material which stimulates the microbial activity in contaminated soil. A great deal of research suggests oil cake as a prospective raw material for the bacterial synthesis of several economically important compounds.19 It is used as organic manure in agriculture fields and contains nutrients for microbial growth. Das et al.20 reported that bioaugmentation coupled with mustard oil cake increased copper remediation in artificially contaminated agriculture soil. Similarly, the application of coconut oil cake increased the Cu bioleaching efficiency of Herbaspirillum sp. GW103.7 However, there are no reports on the application of oil cake extract for immobilization of metals in mine soils.
Pulicat Lake, located in the North Chennai coastal region of India is a typical brackish water ecosystem of great importance with regards to biodiversity and aesthetic value. Previous studies have confirmed the heavy metals such as Hg (2.6 μg g−1), Cr (19.8 μg g−1), Cd (32.7 μg g−1) and Pb (8.32 μg g−1) contamination in the lake.15,21 Hence, the objectives of this study were as follows: (i) isolation and characterization of Pb resistant bacteria from a brackish water environment, (ii) bioaugmentation of Pb contaminated mine soils with bacteria isolated from brackish environment, (iii) biostimulation of non-indigenous bacterial activity using sesame oil cake extract, (iv) sequential extraction of bioremediated mine soil to understand the interaction between Pb resistant brackish environment bacteria and Pb, and (v) estimation of soil metabolic activity after bioremediation.
2. Materials and methods
2.1. Sampling and isolation of Pb resistant bacteria
Sediment samples were collected from 3 different areas of Pulicat Lake using Peterson grab21 transported on ice to the laboratory and processed within 18 h. Previous studies reported the complete physico-chemical characteristics of lake sediments.21,22 Lead resistant bacteria were isolated from the sediment samples according to Kamala-Kannan et al.22 with minor modifications. The serially diluted sediment suspension (0.1 mL) was plated using the spread plate technique onto Luria Bertani (LB) agar (1/4 strength) supplemented with 50 mg L−1 of Pb(NO3)2. Plates were incubated at 25 °C for 2 days and observed for the bacterial growth. Morphologically different colonies were identified, purified, and stored at 4 °C for further study. Isolation and purification of the isolates were carried out at the Department of Applied Geology, University of Madras, India.2.2. Minimal inhibitory concentration of metals
Minimal inhibitory concentration (MIC) of metals was determined by agar dilution method.22 Mid log-phase culture of the isolates were aseptically inoculated onto LB agar (1/4 strength) supplemented with increasing concentrations of Pb (50–750 mg l−1). The plates were incubated at 25 ± 2 °C for 24 h and observed for bacterial growth. The concentration of heavy metals that completely inhibited the growth of the bacteria was considered as MIC.2.3. Genomic DNA extraction and identification of potential isolate SKK11
Cells were harvested from 10 mL of LB broth and lysed in lysis buffer containing 25% sucrose, 20 mM EDTA, 50 mM Tris-HCl, and 5 mg mL−1 lysozyme.23 Chromosomal DNA was extracted according to Maniatis et al.24 The partial 16S rRNA gene was amplified using polymerase chain reaction (PCR) with 27f and 907r primers. The PCR product was purified (QIAGEN, CA, USA) and sequenced using an automated sequencer ABI PRISM (Model 3700, CA, USA). The sequences were compared using BLAST program for the identification of isolates.2.4. Oil cake extraction
Sesame oil cake was procured from a local market in Chennai, India. Chemical composition of the sesame oil cake is presented in Table 1. The oil cake was suspended in sterile ultrapure water (Barnstead NANOpure, Waltham, MA, USA), and the flasks were shaken at constant speed of 150 rpm for 2 h. Later, the mixture was filtered through Whatman no. 1 filter paper followed by 0.2 μm membrane filter. Based on the preliminary studies 2% oil cake extract was used for the experiments.| Chemical components | Quantity (%) | |
|---|---|---|
| Dry matter | 83.2 | Kuo (1967)38 |
| Crude protein | 35.6 | |
| Crude fibre | 7.6 | |
| Ash | 11.8 | |
| Calcium | 2.45 | |
| Phosphorous | 1.11 |
2.5. Growth kinetics of the isolate SKK11
Log phase culture (5 mL) of the isolate SKK11 was aseptically inoculated in LB broth (1/4 strength) supplemented with different concentrations (50, 100, and 150 mg l−1) of Pb. The flasks were incubated in a shaking incubator (180 rpm) at 25 ± 2 °C, and the growth was measured at the prescribed time intervals (12–96 h) in terms of increase in optical density at 600 nm using a UV-Vis spectrophotometer (UV-1800, Shimadzu, Japan). Similarly, another set of experiments were carried out with oil cake extract and Pb (150 mg l−1). Cultures grown in the absence of metal were used as a control (Kamala-Kannan et al. 2006). Results were subjected to two-way analysis of variance (ANOVA) using SPSS software v 12 (Chicago, USA).2.6. Characterization of Pb resistance
2.7. Bioremediation of Pb contaminated mine soil
Exchangeable or bioavailable fraction (F1). Two grams of soil samples were uniformly mixed with 16 mL of 1 M magnesium chloride solution (pH 7.0) and the flasks were incubated in a shaking incubator (40 rpm) at room temperature for 1 h.
Carbonate fraction (F2). The residues from F1 were extracted with 8 mL of 1 M sodium acetate (NaOAc) solution (pH 5.0) with continuous agitation (40 rpm) at 26 °C for 5 h.
Iron and manganese oxide fraction (F3). The residues from F2 were treated with 40 mL of hydroxyl ammonium chloride (HONH2·HCl) (0.04 M in 25% (v/v) acetic acid) for 6 h at 90 ± 2 °C on a hot plate. The samples were periodically agitated.
Organic fraction (F4). The residues from F3 were incubated with 20 mL of 7 M sodium hypochlorite solution (pH 8.5) for 2 h at 90 ± 2 °C on a hot plate. The samples were periodically agitated.
Residual fraction (F5). The residues from F4 were digested with concentrated HNO3 (12 mL) for 2 h at 90 ± 2 °C on a hot plate.
After each extraction (F1–F5), the samples were centrifuged at 6000 rpm for 5 min, the supernatant was acidified with concentrated HNO3, and stored at 4 °C. One milliliter of the supernatant was filtered through a 0.2 μm membrane and analyzed for Pb concentration using inductively coupled plasma mass spectrometry (ICP) (150-00191-1, Rev. A, Leemans labs, USA), after appropriate dilution. The ICP measurement conditions were as follows: Nebulizer gas flow rate: 50 psi; auxiliary gas flow: 16 lpm; plasma gas flow: 16 lpm; ICP RF power: 1.4 kW. Three repetitions were carried out for all the fractions and results were subjected to two-way analysis of variance (ANOVA) using SPSS software v 12 (Chicago, USA).
2.8. Soil enzymes
Urease activity was estimated according to Kandeler.27 Dehydrogenase and alkaline phosphatase activity were estimated according to Tabatabai28 with slight modification in incubation time and the temperature. Briefly, 5 g of the soil samples were mixed with 1 mL of 3% 2,3,5-triphenyltetrazolium and 5 mL of autoclaved water. Later, the samples were vortexed and incubated in dark at 37 °C for 48 h. After incubation, 10 mL of methanol was added, and the samples were shaken for 5 min and filtered. The filtrate was analyzed for triphenyl formazan by spectrophotometric method at 485 nm. Amylase activity was measured according to Galstyan.29 Soil invertase activity was estimated according to Ill et al.30 Soil cellulase activity was estimated according to Kelley and Rodriguez-Kabana.31 Three replications were carried out for all the experiments.3. Results and discussion
3.1. Isolation, identification and heavy metal resistance of SKK11
Seven morphologically different Pb resistant bacterial colonies were isolated from the Pulicat Lake sediments, and the isolates were repeatedly screened for their Pb resistance in 1/4 strength LB agar to prevent Pb precipitation. The isolates were designated as SKK11, SKK12, SKK13, SKK14, SKK15, SKK16, and SKK17. The results of the MIC showed that isolate designated SKK11 was the most resistant to Pb (750 mg l−1). Thus, the isolate SKK11 was selected for further studies. The results are consistent with previous studies reporting Pb resistance in bacteria isolated from the sediments of Pulicat Lake.15 However, the MIC of the isolate SKK11 appears to be higher than the previous isolates. Several reasons may explain the differences in metal resistance range. The mode of metal resistance may differ from previous isolates. Alternatively, medium strength, chemical composition of the medium and nature of the medium influences the bioavailability of metals resulting in a difference in MICs for metals. The 16S rDNA sequence of this strain showed 99% identity with Bacillus sp. (GenBank Accession no. FJ946999).3.2. Growth studies
Growth response of the isolate SKK11 in the presence of different concentrations of Pb is presented in Fig. 1. A limited difference in the lag phase observed in the presence of Pb, which could be due to the Pb toxicity. The results are consistent with previous studies reporting the difference in growth rates of the Bacillus sp. in the presence of metals. Similarly, growth of the isolate SKK11 in the presence of oil cake extract (2%) was evaluated, and the results are shown in Fig. 1. Extended log phase was observed in the presence of oil cake extract, which could be due to availability of more nutrients and reduced toxicity of metals. The results are in agreement with previous studies reporting a significant increase of bacterial growth on co-incubation with oil cake amended contaminated soil.203.3. Characterization of Pb resistance
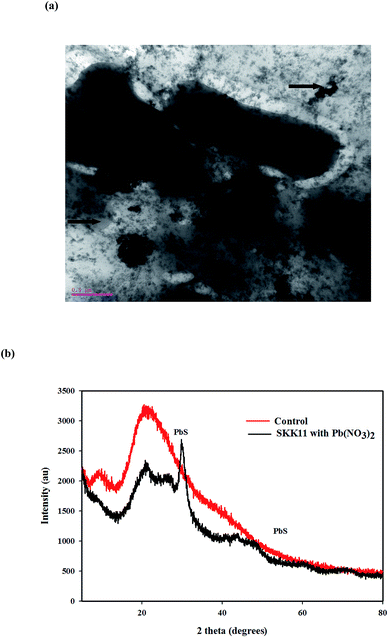
A transmission electron micrograph and XRD spectra of the isolate SKK11 are shown in Fig. 2(a and b). The results revealed that isolate SKK11 transformed Pb(NO3)2 into PbS. Pb particles was visible as dark granules on outside the bacterial cells. The isolate may transform Pb(NO3)2 to PbS either via oxidative or reductive mechanisms.32 Extracellular proteins, phospholipids, organic acids and enzymes could be involved in the transformation of Pb.11 The transformation of Pb(NO3)2 or PbCl2 into PbS nanoparticles has been reported before for the phototrophic bacterium Rhodobacter sphaeroides.33 The isolate was screened for pbrT gene, a membrane Pb transport protein reported in the genus Bacillus. No visible band was observed on the gel, which indicates that the isolate SKK11 may harbor another type of Pb transporter protein or that the primers (pbrTf and pbrTr) were inappropriate for the amplification of the pbrT gene. Alternatively, the isolate SKK11 may transform the Pb(NO3)2 extracellularly.253.4. Soil remediation studies
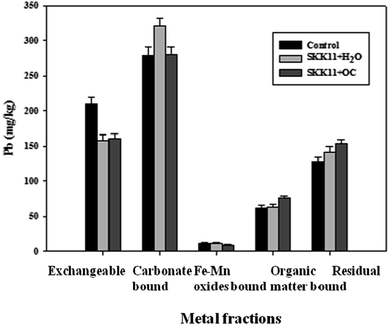
Pb immobilization efficiency of the isolate SKK11 in the presence of oil cake was determined by the sequential extraction methods, and the results are presented in Fig. 3. The total concentration of Pb (687.2 mg kg−1) can be used as a general index for soil pollution, and it does not provide information about the different fractions of Pb and bacteria-Pb interactions. To provide a comprehensive picture of different Pb fractions and Pb-bacteria interactions, the Pb concentration in mine soils was determined by sequential extraction methods. Five different fractions, such as exchangeable, carbonate, Fe–Mn oxides, organic, and residual fractions were determined by these sequential extraction methods. The order of Pb distribution was carbonate > exchangeable > residual > organic > Fe–Mn oxide. Marked difference in Pb distribution on exchangeable, carbonate, and residual fractions was observed in control and bioremediated mine soils. The two-way ANOVA analysis showed that the Pb distribution significantly differed at 5% in soil treatment, fraction and soil treatment vs. fraction as a factors. The results are consistent with previous studies reporting the significant variation in metal fraction after bioaugmentation.34The exchangeable fraction of Pb in mine soils was 209.5 mg kg−1 and accounted for 30.5% of total Pb concentration. However, a decrease in exchangeable Pb fraction (22.9% in SKK11 augmented soil and 24.9% in SKK11 + oil cake extract augmented soil) was observed in bioremediated soil. The nutrients present in the oil cake extract may enhance the activity of the isolate SKK11 in biostimulated soil. The results indicate that isolate SKK11 transformed exchangeable fraction into non-bioavailable form.13,35 However, 75.1% of exchangeable fraction remained in the mine soil and it could due limited incubation time. Alternatively, bioaugmentation with microbial consortium or coupling of bioaugmentation with conventional chemical process may completely immobilize the exchangeable fraction of metals in contaminated soil.
The carbonate fraction of Pb in mine soils was 278.6 mg kg−1 and accounted for 40.6% of total Pb concentration. However, a considerable increase (15.3%) in the carbonate fraction was observed in the bioremediated mine soil which was treated with the isolate SKK11. The results further confirm the potential of the isolate SKK11 on transformation of Pb in mine soils. The increased distribution of carbonate-bound Pb was due to the bacteria-induced carbonate precipitation. The role of bacteria induced calcite precipitation on transformation of metals is well established in several studies.35,36 The results corroborate with the studies by Achal et al.13 and Govarthanan et al.34 reported a significant increase in carbonate fraction of metals after bioaugmentation. Conversely, the distribution of carbonate fraction was not increased in bioremediated soil which was amended with oil cake extract. Several reasons may explain the differences in carbonate fraction of Pb among bioremediated soils. The presence of oil cake extract may alter the geochemical conditions of the mine soils and thereby the formation of calcite precipitates. Alternatively, the oil cake extract may alter the interactions of bacterial metabolic products and ions or compounds involved in the calcite precipitation. This was supported by the results from XRD studies where the intensity of calcite peaks in oil cake amended bioremediated soil was similar to control soil (Fig. 4).
The distribution of Fe–Mn oxide fraction was not altered in control and bioremediated soils. The results indicate that isolate SKK11 did not interact with Fe–Mn oxide fraction. Several reasons may explain the inefficiency of the isolate SKK11 to interact with Fe–Mn fraction; it is well known that soils are the ‘sinks’ for heavy metals. Alternatively, the metal present in the Fe–Mn oxides may not be readily exchangeable for the isolate SKK11. The results are consistent with previous studies reporting that bioaugmentation did not significantly reduce Fe–Mn oxide fraction of metals.13,34
The distribution of organic matter bound Pb in the control soil was 61.21 mg kg−1 and accounted for 9.1% of total Pb concentration. The concentration was not altered in bioremediated soil which was not amended with oil cake extract. The results showed that isolate SKK11 was not interacted with organic fraction of Pb because it is not be readily bioavailable.22 However, an increase (23.5%) in the organic matter bound Pb fraction was observed in bioremediated soil which was amended with oil cake extract. The organic matters present in the oil cake extract may interact with the available Pb and increase the distribution of organic bound Pb.20
On average, the distribution of Pb associated with residual fraction accounted for 18.5% of total Pb present in the mine soils. However, an increase (21.2%) in the residual fraction was observed on oil cake amended bioremediated soil. The increased distribution of F5 fraction in bioremediated soil was due metal transformation and it further confirms the potential of the isolate SKK11. The results are in accordance with Varenyam et al.35 reporting a significant increase in the residual fraction of Pb after bioremediation using Kocuria flava. The results of the fraction studies indicate that the isolate SKK11 effectively interacted with the exchangeable fraction of Pb and alleviates Pb mobilization in mine soils. The X-ray diffractograms of soils are presented in the Fig. 4. The results confirmed the presence of various minerals, such as calcite, aragonite, halite, quartz, plagioclase, and gwihabaite in the mine soils. Quartz, calcite and plagioclase dominated the mineralogy profile in mine soil samples. However, a significant decrease (79.0%) in the intensity of the plagioclase peak was observed in the bioremediated mine soils amended with oil cake extract. The extracellular metabolic products and activity of the isolate SKK11 may degenerate the plagioclase peak in bioremediated soil. The results are consistent with previous study reporting the role of microbial extra cellular polysaccharides in plagioclase mineral dissolution.36 Nowadays, bioaugmentation coupled with biostimulation is believed to be one of the most-effective methods for simultaneously increasing metal removal and soil fertility besides other bioremediation methods. The poor survival of the microorganisms in the metal contaminated soil is enhanced by direct addition of oil cake into the soil. A deeper understanding of microbial lifestyle and dynamics of communities found in biostimulated soil is thus necessary to further increase the effect of oil cake on remediation of contaminated soils.
3.5. Soil enzymes
The enzyme activity of the bioremediated soil is shown in the Table 2. A marked increase in the enzyme activities was observed in oil cake amended bioremediated soil, which indicates the potential role of the isolate SKK11 and oil cake extract on metabolic recovery of mine soils. The results have further confirmed that presence of oil cake extract increase the growth and activity of the isolate SKK11 in mine soils. The bioremediation coupled with oil cake extract amendment increased the extracellular enzyme activity and, thereby, the metabolic activity of the mine soils. The results are in agreement with several studies reporting the correlation between microbial activity and soil enzyme activity.37| S. no | Enzymes | Control | SKK11 | SKK11 + OC |
|---|---|---|---|---|
| 1 | Amylase (mg glucose per g 2 h) | 35 ± 1.4 | 60 ± 2.1 | 110 ± 2.8 |
| 2 | Cellulase (mg glucose per g 2 h) | 35 ± 2.1 | 70 ± 1.4 | 85 ± 2.1 |
| 3 | Dehydrogenase (mg TPF per g soil) | 45 ± 0.7 | 60 ± 2.8 | 100 ± 2.1 |
| 4 | Invertase (mg glucose per g 2 h) | 25 ± 0.7 | 45 ± 2.1 | 85 ± 0.3 |
| 5 | Phosphatase (U per g dry soil) | 40 ± 2.1 | 80 ± 2.1 | 160 ± 1.8 |
| 6 | Urease (mg N g−1 soil per 2 h) | 35 ± 1.4 | 64 ± 1.4 | 93 ± 0.2 |
4. Conclusion
The metal resistant bacteria Bacillus sp. SKK11 isolated from brackish environment was capable of immobilizing Pb in mine soils. The bioaugmentation coupled with biostimulation immobilized 24.9% of exchangeable fraction and increased the metabolic activity of the mine soil. The observations indicate the potential role of the isolate SKK11 and oil cake extract for bioremediation process. Further work will address the interactions between the selected bacterium and minerals, and fertility of the bioremediated soil as well as for improvement of the efficiency of lead conversion from available into non-available fractions.Acknowledgements
This research work was supported by the National Research Foundation of Korea (NRF) grant funded by the government (MEST; no. 2011-0020202).References
- S. Khan, Q. Cao, H. Abd El-Latif, X. Yue and H. E. Ji-Zheng, J. Environ. Sci., 2007, 19, 834–840 CrossRef CAS.
- M. Valls and V. D. Lorenzo, FEMS Microbiol. Rev., 2002, 26, 327–338 CrossRef CAS PubMed.
- L. Y. He, Z. J. Chen, G. D. Ren, Y. F. Zhang, M. Qian and X. F. Sheng, Ecotoxicol. Environ. Saf., 2009, 72, 1343–1348 CrossRef CAS PubMed.
- J. E. Yang, Y. S. Ok, W. I. Kim and J. S. Lee, Heavy metal pollution, risk assessment and remediation in paddy soil environment: research and experiences in Korea, in Cause and Effects of Heavy Metal Pollution, Nova Science Publishers, New York, 2008 Search PubMed.
- G. Borbely and E. Nagy, Desalination, 2009, 240, 218–226 CrossRef CAS.
- Y. S. Ok, S. C. Kim, D. K. Kim, J. G. Skousen, J. S. Lee, Y. W. Cheong, S. J. Kim and J. E. Yang, Environ. Geochem. Health, 2011, 33, 23–30 CrossRef CAS PubMed.
- M. Govarthanan, G. W. Lee, J. H. Park, J. S. Kim, S. S. Lim, S. K. Seo, M. Cho, H. Myung, S. Kamala-Kannan and B. T. Oh, Chemosphere, 2014, 109, 42–48 CrossRef CAS PubMed.
- A. Walton, L. Ebdon and G. Millward, Appl. Organomet. Chem., 1988, 2, 87–93 CrossRef.
- P. T. S. Wong, Y. K. Chau and P. L. Luxon, Nature, 1975, 253, 263–264 CrossRef CAS PubMed.
- J. S. Thayer, Appl. Organomet. Chem., 2002, 16, 677–691 CrossRef CAS.
- M. K. Guria, A. K. Guha and M. Bhattacharyya, J. Environ. Chem. Eng., 2014, 2, 424–433 CrossRef CAS.
- A. Mrozik and Z. Piotrowska-Seget, Microbiol. Res., 2010, 165, 363–375 CrossRef CAS PubMed.
- V. Achal, X. Pan, Q. Fu and D. Zhang, J. Hazard. Mater., 2012, 202, 178–184 CrossRef PubMed.
- A. Braud, K. Jezequel, S. Bazot and T. Lebeau, Chemosphere, 2009, 74, 280–286 CrossRef PubMed.
- S. Kamala-Kannan and R. Krishnamoorthy, Sci. Total Environ., 2006, 367, 341–353 CrossRef PubMed.
- K. R. Reddy, S. Chinthamreddy, R. E. Saichek and T. J. Cutright, Energy Sources, 2003, 25, 931–943 CrossRef CAS.
- T. M. Roane, Microb. Ecol., 1999, 37, 218–224 CrossRef CAS PubMed.
- T. Wang, H. Sun, H. Mao, Y. Zhang, C. Wang, Z. Zhang, B. Wang and L. Sun, J. Hazard. Mater., 2014, 278, 483–490 CrossRef CAS PubMed.
- S. Ramachandran, S. K. Singh, C. Larroche, C. R. Soccol and A. Pandey, Bioresour. Technol., 2007, 98, 2000–2009 CrossRef CAS PubMed.
- C. Das, A. Bhowal and S. Datta, Biorem. J., 2011, 15, 90–98 CrossRef CAS.
- S. Kamala-Kannan, B. P. D. Batvari, K. J. Lee, N. Kannan, R. Krishnamoorthy, K. Shanthi and M. Jayaprakash, Chemosphere, 2008, 71, 1233–1240 CrossRef CAS PubMed.
- S. Kamala-Kannan, S. Mahadevan and R. Krishnamoorthy, Arch. Microbiol., 2006, 185, 202–211 CrossRef PubMed.
- B. T. Oh, H. Hur, K. J. Lee, K. Shanthi, B. Y. Soh, W. J. Lee, H. Myung and S. Kamala-Kannan, Biocontrol. Sci. Technol., 2011, 21, 1297–1311 CrossRef.
- T. Maniatis, E. F. Fritsch and J. Sambrook, Molecular Cloning: A Laboratory Manual, 2nd edn, 1989 Search PubMed.
- M. N. Shin, J. Shim, Y. You, H. Myung, K. S. Bang, M. Cho, S. Kamala-Kannan and B. T. Oh, J. Hazard. Mater., 2012, 199−200, 314–320 CrossRef CAS PubMed.
- Y. C. Song, S. Sivakumar, T. T. Nguyen, S. H. Kim and B. G. Kim, J. Hazard. Mater., 2009, 167, 1033–1037 CrossRef CAS PubMed.
- E. Kandeler, Methods in Soil Biology, Springer-Verlag, Heidelberg, New York, 1996, pp. 171–174 Search PubMed.
- M. A. Tabatabai, Soil Enzymes, 1994, 775–833 Search PubMed.
- A. S. Galstyan, Eurasian Soil Sci., 1965, 2, 170–175 Search PubMed.
- F. G. Ill, C. A. Clausen and T. L. Highley, Anal. Biochem., 1989, 182, 197–199 CrossRef.
- W. D. Kelley and R. Rodriguez-Kabana, Can. J. Microbiol., 1975, 21, 565–570 CrossRef CAS PubMed.
- G. M. Gadd, Microbiology, 2010, 156, 609–643 CrossRef CAS PubMed.
- H. J. Bai and Z. M. Zhang, Mater. Lett., 2009, 63, 764–766 CrossRef CAS.
- M. Govarthanan, K. J. Lee, M. Cho, J. S. Kim, S. Kamala-Kannan and B. T. Oh, Chemosphere, 2013, 90, 2267–2272 CrossRef CAS PubMed.
- A. Varenyam, X. Pan, D. Zhang and Q. Fu, J. Microbiol. Biotechnol., 2012, 22, 244–247 CrossRef.
- X. L. Pan, Res. J. Chem. Environ., 2009, 13, 3–4 Search PubMed.
- S. A. Welch, W. W. Barker and J. F. Banfield, Geochim. Cosmochim. Acta, 1999, 63, 1405–1419 CrossRef CAS.
- L. H. Kuo, Malays. Agric. J., 1967, 46, 63–70 Search PubMed.
Footnote |
| † The first two authors made equal contribution to this work. |
| This journal is © The Royal Society of Chemistry 2015 |