A 3-D metal–organic framework with a rutile topology network, right- or left- handed helical chains and tunable UV-to-visible photoluminescence†
Abstract
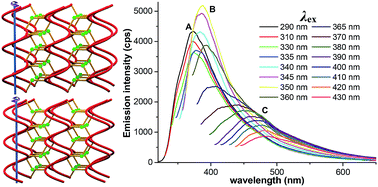
A rutile topological 3-D metal organic framework (MOF) has been obtained by reacting CdSO4·8/3H2O with HL (HL = 6-aminonicotinic acid) under solvothermal conditions. Unique right- or left- handed helices can be found in the architecture of the MOF. The framework can exhibit tunable UV-to-visible fluorescence by variation of the excitation light.

 Please wait while we load your content...
Please wait while we load your content...