Discovery of a low affinity thyrotropin-releasing hormone (TRH)-like peptide that exhibits potent inhibition of scopolamine-induced memory impairment in mice†
Abstract
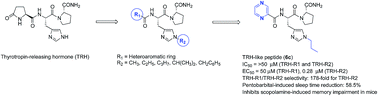
TRH-like peptides were synthesized in which the critical N-terminus residue L-pGlu was replaced with various heteroaromatic rings, and the central residue histidine with 1-alkyl-L-histidines. All synthesized TRH-like peptides were evaluated in vitro as agonists in HEK mTRH-R1 and HEK mTRH-R2 cell lines, an expressing receptor binding assay (IC50), and cell signaling assay (EC50). The analeptic potential of the synthesized peptides was evaluated in vivo by using the antagonism of a pentobarbital-induced sleeping time. The peptides 6a, 6c and 6e were found to activate TRH-R2 with potencies (EC50) of 0.002 μM, 0.28 μM and 0.049 μM, respectively. In contrast, for signaling activation of TRH-R1, the same peptides required higher concentration of 0.414 μM, 50 μM and 19.1 μM, respectively in the FLIPR assay. The results showed that these peptides were 207, 178 and 389-fold selective towards TRH-R2 receptor subtype. In the antagonism of a pentobarbital-induced sleeping time assay, peptide 6c showed a 58.5% reduction in sleeping time. The peptide 6c exhibited high stability in rat blood plasma, a superior effect on the scopolamine-induced cognition impairment mice model, safe effects on the cardiovascular system, and general behavior using a functional observation battery (FOB).

 Please wait while we load your content...
Please wait while we load your content...