Morphological evolution of gold nanoparticles on silicon nanowires and their plasmonics†
Abstract
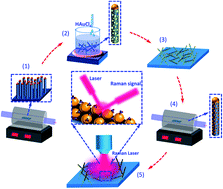
One-dimensional heterostructures composed of silicon (Si) nanowires and uniformly decorated with gold (Au) nanoparticles were fabricated and used as a substrate for organic detection based on the surface-enhanced Raman spectroscopy. Si nanowires were grown via a silane-based chemical vapor deposition approach. The controlled decoration of Au nanoparticles on the Si nanowires was achieved using wet-chemical nucleation and followed by a thermal treatment process. Various annealing parameters were studied to control the shape, size, and surface dispersion of Au nanoparticles on the heterostructures. Microscopic methods were used to evaluate the configuration or morphological evolution (size, inter-particle spacing and density) of nanoparticles at different annealing conditions. The influence of annealing on the chemical composition of Au nanoparticles were analyzed using X-ray photoelectron spectroscopy (XPS) and the phase transformation kinetics was also studied. Finally, surface-enhanced Raman spectroscopy was used to sense organic species and this was studied for various morphologies of nanowire heterostructures.

 Please wait while we load your content...
Please wait while we load your content...