Multivariate interactions of natural and anthropogenic factors on Cd behavior in arable soil
Fangli Wangab,
Wei Ouyang*a,
Fanghua Haoa,
Andrea Crittob,
Xuchen Zhaoa and
Chunye Lina
aState Key Laboratory of Water Environment Simulation, School of Environment, Beijing Normal University, Beijing 100875, P. R. China. E-mail: wei@bnu.edu.cn; Fax: +86 10 5880 2078; Tel: +86 10 5880 2078
bDepartment of Environmental Sciences, Informatics and Statistics, University Ca' Foscari, Calle Larga S. Marta 2137, I-30123 Venice, Italy
First published on 30th April 2015
Abstract
Multivariate interactions are far more complex between natural factors and pollutants resulting from anthropogenic practices than between pollutants themselves. But little attention has been focused on the complex interpretation of multivariate interactions. To bridge this research gap, this study aimed to identify the interactive effect of multiple affecting factors including freeze–thaw cycles (FT), soil water (SW) content, and chlorpyrifos (CP) on soil Cd behavior in arable soil, based on the analysis of changes in Cd fractionations and Cd availability. Moreover, the significant effect was computed via design of experiments. The content of Cd fractions and the evaluated index of Cd availability were obtained by employing the modified six-step sequential extraction method. The results showed that the main effect of FT, SW and CP on Cd fractionation and availability was significant. The binary interactions weakened the main effect of FT or SW, but enhanced the main effect of CP on Cd fractionation. The ternary interactions further weakened the binary interactions, whereas CP enhanced the interaction between SW and FT. The interaction between SW and CP had a negative effect on residual Cd, but positively affected water-soluble and organic matter-bound Cd. The binary interaction between CP and FT had a positive effect on residual Cd (21.0%), but negatively affected water-soluble and Fe–Mn-oxide-associated Cd (25.9% and 21.1%). These results covered more innovative information on the multivariate interactions between natural and anthropogenic factors on Cd behavior in arable soil. A possible new way to quantify the significant impact of multivariate factors also was provided.
1. Introduction
Anthropogenic practices have generally caused the release of heavy metals (HMs) and pesticides in the environment. The interactions between HMs and pesticides in arable soil have caused world-wide concern, as they have been linked to many ecological and human health risks.1 Previously, such interactions (mainly including synergism and antagonism) have been reported in many reports.2 For instance, synergism was observed in the binary mixture of Cd and dimethoate,1 while antagonism was discovered showing that chlorpyrifos could reduce the toxicity of nickel by competing for the same binding sites.3 The effect of copper-carbendazim on the reproduction of Caenorhabditis elegans was synergistic at low dose levels but antagonistic at high dose levels.4 The aforementioned studies focusing on the interactions between HMs and pesticides are mostly investigated in laboratory experiments under well-controlled conditions.2 However, the actual field conditions are much more complicated than in the laboratory due to the large fluctuations in natural environmental factors. Moreover, the impact of natural environmental factors on the interactions between HMs and pesticides are more serious than that of pollutants themselves.5 Nevertheless, studies addressing this topic are really scarce.Seasonal freeze–thaw (FT) is fairly common in mid-high latitude regions among these natural factors. FT and soil water content (SW) play an import role in controlling the mass transport and energy exchange in the soil-plant-atmosphere-climate system.6 Also, both FT and SW can alter soil physio-chemical and biological properties,5,7 such as soil pH, the dissolved organic matter (DOM) and the activity of soil organisms, which are easily affected by pesticides.8 At least two of these properties are usually observed to control HMs behavior.9 For instance, FT can destroy soil aggregates, and then increase the content of DOM, which will subsequently alter the capacities of soil to bind HMs.10,11 The increased SW can enhance the extent to which soil aggregates are damaged by FT, which will decrease when SW exceeds the saturation value.12 A significant increase in the total amount of free amino acids and sugars caused by freezing is combined with an increment in soil respiration and dehydrogenase activities,13 which can easily be affected by pesticides.14 The above phenomenon indicates that interactions between the two natural factors (FT and SW) and pesticides are far more complex in the mechanism, which may directly/indirectly further affect the behavior of pollutants themselves. Therefore, a scientific basis should also be provided to consider the impact of the natural environmental factors, especially on that how natural stressors interact with chemical stressors. Further researches are desired to conduct focusing on the elaborate experimental design and the complex interpretation of multivariate interactions.2
Design of experiments (DOE) has been reported to provide maximized information of experimental statistics and to get unambiguous results taking minimized efforts.15 2n full factorial design in DOE is a statistical technique for designing experiments where n affecting factors are controlled.16 The effects of various affecting factors are investigated at each of two levels, containing the minimal and the maximal input–output data pairs.17 On this basis, the effect of main effects and interactions is assessed by the outputs viz the values of estimated effect (E). A higher E reveals a stronger effect. The main effect reveals the impact of one changed controlling factor, while the interaction reveals the combined impact of the multivariate affecting factors.16 This method has been widely used in behavior sciences to analyse a random response of output variables to a set of various affecting factors.
Therefore, to bridge the research gap in multivariate interactions between natural and anthropogenic factors on HMs behavior, based on the analysis of changes in HMs fractionations and HMs availability, this study aims to: (1) evaluate the potential binary and ternary interactions between natural factors (FT and SW) and pesticides on HMs behavior in arable soil; (2) provide a possible way to quantify the significant effect of these affecting factors on the basis of DOE analysis. Cd and chlorpyrifos (CP) were selected as the representatives due to their intrinsic properties, such as the tendency of accumulation,18 the most toxic and mobile in arable soil19 and the special chemical structure to inhibit the activity of acetylcholinesterase.8,14 Cd fractionation and Cd availability were selected to investigate because they have been reported to provide valuable information on represent Cd behavior.9 Soil samples were collected from an arable soil in a typical seasonal frozen region of northeast China. A 23 full factorial design was carried out based on the hypothesis that Cd behavior was influenced by the interactive effects of FT, SW and CP.
2. Materials and methods
Soil sampling
The location of sampling site was illustrated in Fig. 1. Soil samples were collected from an arable soil located in northeastern China (47°24′N, 134°05′E), where soils were regularly exposed to sub-zero temperatures for approximately six months.20 The minimum and maximum air temperatures of the studied area were −20.2 °C in January and 21.7 °C in July. The average depth of the frozen soil was 141 cm.6 Rain-fed agriculture was the dominant production system of the study maize-field region,20 and CP was widely applied to corn and soybean in this seasonal frozen region. The total Cd in the soil has been reported to present in a trend of accumulation due to the long-term fertilization of rock phosphate and application of pesticides.18 Surface soils (<20 cm) were collected with five sampling replicates in a “W” shape. All five soil replicates were blended, air-dried and ground.Experimental preparation
Parts of soil samples were sieved with a 0.147 mm nylon sieve. The soil pH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5, soil
2.5, soil![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water) was determined using a combination electrode. The maximal water holding capacity was determined following the method described by Muhammad et al.21 After the soil saturation and later drainage in a water-saturated atmosphere, the water content was determined. The soil was a silt loam and classified as burozem,22 with a maximal water holding capacity of 41.2% and a pH of 5.68.
water) was determined using a combination electrode. The maximal water holding capacity was determined following the method described by Muhammad et al.21 After the soil saturation and later drainage in a water-saturated atmosphere, the water content was determined. The soil was a silt loam and classified as burozem,22 with a maximal water holding capacity of 41.2% and a pH of 5.68.
Prior to Cd analysis, a HF–HNO3–HClO4 acid mixture was used for the digestion of the ground soil and the constant-volume acid mixture was determined by inductively coupled plasma optical emission spectrometry (ICP-OES, IRIS Intrepid II XSP, Thermo Electron, USA). The concentration of total Cd in the soil was 0.009 mg kg−1.
Parts of soil samples were sieved with a 2 mm nylon sieve. 1 kg of soil was weighed into a plastic box. 10 mg L−1 of Cd2+ solution was added into the soil with a volume of 1 L. To homogenize Cd in soil, the mixture was incubated under a room temperature for approximately 2 months. The total content of Cd in soil thereby was adjusted to 10 mg kg−1. Then, the soil was air-dried and sieved by a 2 mm mesh sieve again and subsample specimens (1 g each) were weighed in a plastic bag.
Experimental design
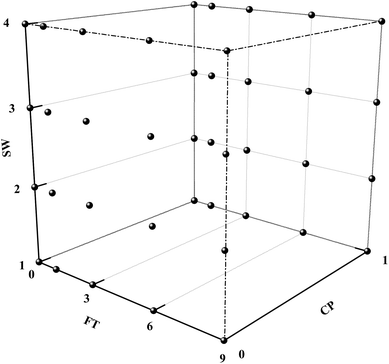
As shown in Fig. 2, the study was conducted in a completely randomised design with four levels of soil water content (SW1-70%, SW2-100%, SW3-120% and SW4-150% of the maximal water holding capacity) and two levels of CP (CP0 and CP1, 0 and 5 mg kg−1). The subsamples were subsequently subjected to the FT process with a frequency of 1 (F1), 3 (F3), 6 (F6) or 9 (F9). Meantime, the subsamples kept under the thawing temperature was set as the control group (F0). Each treatment was replicated four times. The total number of the experimental groups and samples was 40 and 160, respectively. Freezing and thawing were carried out over a period of 24 hours each (the FT frequency was set at 2 days) and the soil temperature was set in accordance with the air temperature. In order to simulate field conditions, this study accordingly used a freezing temperature of −10 °C and a thawing temperature of 20 °C. This was based on the fact that the soil temperature exceeded the air temperature by approximately 5–10 °C when the surface layer of the soil began to freeze.23Modified six-step sequential extractions and Cd availability calculation
Cd fractionations were determined using modified Tessier six-step sequential extractions method, which was reported in 2007.24 Soil samples were sequentially extracted by (1) deionised water for 1 h (20 °C); (2) 1 M NH4OAc (pH 7.0) for 2 h; (3) 1 M NH4OAc (pH 5.0) for 2 h; (4) 0.04 M NH2OH–HCl in 25% HOAc for 6 h (water bath, 60 °C); (5) 30% H2O2 (pH 2, adjusted with HNO3) for 5.5 h (water bath, 80 °C) + 3.2 M NH4OAc in 20% HNO3 for 30 min, and (6) HF–HNO3–HClO4 acid mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); in order to obtain six fractions: water-soluble Cd (Wat-Cd), exchangeable Cd (Exc-Cd), carbonate-bound Cd (Car-Cd), Fe–Mn-oxide-associated Cd (Oxi-Cd), organic matter-bound Cd (Org-Cd) and residual Cd (Res-Cd), respectively.24 The Cd concentrations in all extracts were determined by ICP-OES, with an average recovery of 98.4 ± 5%. Triplicate samples were analysed, and the data were expressed as the means. The results showed that the standard error was within 10%. The content of each fraction of Cd in soil was calculated as the ratio of each fraction to the sum of the extracted Cd in all six-sequential fractions.
2); in order to obtain six fractions: water-soluble Cd (Wat-Cd), exchangeable Cd (Exc-Cd), carbonate-bound Cd (Car-Cd), Fe–Mn-oxide-associated Cd (Oxi-Cd), organic matter-bound Cd (Org-Cd) and residual Cd (Res-Cd), respectively.24 The Cd concentrations in all extracts were determined by ICP-OES, with an average recovery of 98.4 ± 5%. Triplicate samples were analysed, and the data were expressed as the means. The results showed that the standard error was within 10%. The content of each fraction of Cd in soil was calculated as the ratio of each fraction to the sum of the extracted Cd in all six-sequential fractions.
The Cd availability in soils was usually determined by the potential bioavailability and mobility of Cd. The potential Cd bioavailability reflected the portion of the total Cd that could be taken up directly or indirectly by organisms in the soil. On this basis, the index of potential Cd bioavailability was obtained using the following equation: K (%) = (Wat-Cd + Exc-Cd + Car-Cd)/(Wat-Cd + Exc-Cd + Car-Cd + Oxi-Cd + Org-Cd + Res-Cd), where K was the relative content of the potential bioavailable fractions including the sum of Wat-Cd, Exc-Cd and Car-Cd.25 The potential Cd mobility in soil usually indicated the absolute and the relative content of fractions weakly bound to soil components.21 Therefore, the relative index of potential Cd mobility was calculated using the following equation: M (%) = (Wat-Cd + Exc-Cd)/(Wat-Cd + Exc-Cd + Car-Cd + Oxi-Cd + Org-Cd + Res-Cd), where M was the relative content of the potential mobile fractions including the sum of Wat-Cd and Exc-Cd.
Quality assurance and quality control were assessed using duplicates, method blanks and standard reference materials (GBW07401) from the Chinese Academy of Measurement Sciences for each batch of samples. All chemicals were of analytical grade or better, and all glassware and centrifuge tubes were previously soaked in acid (10% HNO3) and rinsed with deionised water.
Statistical analysis
Descriptive data and statistical analyses were carried out using Minitab v.16.0 and Origin 8.0 software, providing the means ± S.D. (standard deviation) of four replicates. A variance analysis of all the data was carried out by the general linear model (GLM) using SPSS v.16.0 with a significant level of 0.05. This model defined the relationship between a dependent continuous outcome variable (six Cd fractions and two availability indexes) and multiple independent variables (5-level FT, 4-level SW and 2-level CP).26 It performed ANOVA by using the least squares regression to fit general linear models.27 In the procedure, the relationships between dependent and independent variables were also given as the main effects and the interactions, similar to the case in the DOE analysis. But the magnitude of the impact was evaluated by the partial eta squared values (eta) of between-subjects in the GLM process.28 In detail, a higher value of the eta referred to a stronger effect. When the value of the eta was greater than or equal to 0.14 or 0.06, the impact was considered to be strong or moderate, respectively.Furthermore, a 23 full factorial design was carried out to identify the impact of affecting factors (FT, SW and CP) on Cd fractionation and Cd availability. Based on the output results, the contribution rate of various factors as well as their higher-order interactions was calculated using the following equation: Ri (%) = Ei/(E1 + E2 +…), where Ri was the relative percentage of the i th estimated effect (E) of the significantly positive/negative factor in the sum of the estimated effects of all the significantly positive/negative factors.
3. Results
Cd fractionation and Cd availability in soil response to FT, SW and CP manipulations
As indicated in Fig. 3, Exc-Cd (22.0–33.1%) and Res-Cd (21.9–41.3%) were the predominant fractions in the soil, while small amounts of Wat-Cd (0.25–1.48%) and Org-Cd (2.53–7.02%) were also detected. Wat-Cd reached the maximum almost two FT cycles earlier in soils with CP than in soils without CP, while the minimal Car-Cd and the maximal Res-Cd emerged two or three FT cycles later. Interestingly, the irregular distribution changes in the soil were observed due to the variation in SW content as a function of FT and CP. In particular, the content of Wat-Cd, Exc-Cd and Org-Cd was significantly (P < 0.05) increased by circa 16.0%, 4.67% and 8.93% due to the addition of CP, respectively, whereas the content of Oxi-Cd was significantly (P < 0.05) decreased by circa 10.8%.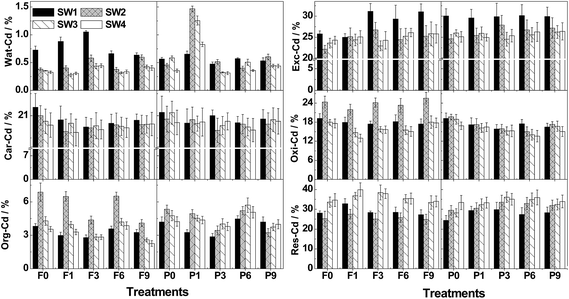 | ||
| Fig. 3 Percentage distribution of Cd in each fraction of the six-step sequential extraction for variously treated soils. | ||
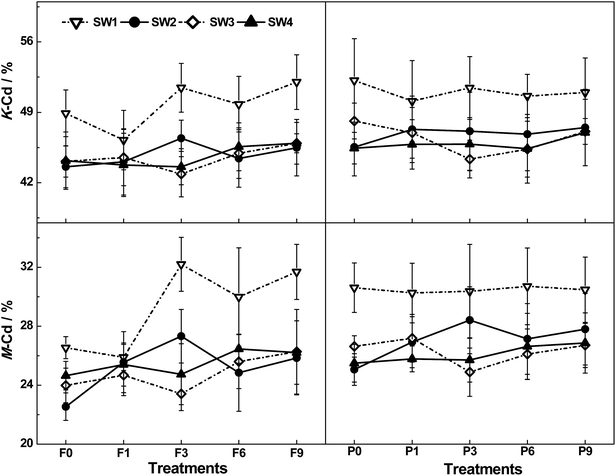
The K values slightly decreased to a minimum at F1, then gradually increased as a result of the increased FT frequency. A notable decrease in the K and M values (approximately 10.3% and 14.7%, respectively) was observed as SW content increased. However, there was no significant variation once the saturation value was exceeded. It was five FT cycles earlier for the minimal K value emerging due to the addition of CP, which also caused the K and M values significantly (P < 0.05) to increase by circa 3.49% and 4.96%, respectively (Fig. 4, Table 1).
| Treatments | N | Wat-Cd | Exc-Cd | Car-Cd | Oxi-Cd | Org-Cd | Res-Cd | K | M |
|---|---|---|---|---|---|---|---|---|---|
| a Different small and capital letters in the same column indicate significant differences between the freeze–thaw and water content treatments, respectively (P < 0.05). Asterisk indicates that there is a significant difference relative to the data before adding CP (P < 0.05). | |||||||||
| F0 | 32 | 0.47 ± 0.14c | 25.2 ± 2.40c | 20.8 ± 1.98a | 19.2 ± 2.48a | 4.63 ± 1.08b | 29.6 ± 4.45c | 46.5 ± 3.60a | 25.7 ± 2.48c |
| F1 | 32 | 0.76 ± 0.42a | 25.7 ± 2.07bc | 19.6 ± 1.87b | 16.9 ± 2.84c | 4.23 ± 1.12c | 32.8 ± 4.46a | 46.1 ± 3.38a | 26.5 ± 2.15bc |
| F3 | 32 | 0.52 ± 0.22b | 26.6 ± 3.25ab | 19.5 ± 1.54b | 16.9 ± 3.09c | 3.37 ± 0.67d | 33.0 ± 5.30a | 46.6 ± 3.86a | 27.1 ± 3.38ab |
| F6 | 32 | 0.44 ± 0.12d | 26.7 ± 2.76ab | 19.4 ± 1.39b | 16.5 ± 3.36c | 4.82 ± 1.00a | 32.0 ± 4.76ab | 46.6 ± 3.35a | 27.2 ± 2.83ab |
| F9 | 32 | 0.51 ± 0.09b | 27.2 ± 2.81a | 20.0 ± 1.48ab | 18.0 ± 3.30b | 3.42 ± 0.74d | 30.8 ± 4.15bc | 47.7 ± 3.33a | 27.7 ± 2.85a |
| SW1 | 40 | 0.67 ± 0.17A | 29.2 ± 2.77A | 20.5 ± 1.76A | 17.7 ± 1.73B | 3.54 ± 0.64D | 28.4 ± 3.04B | 50.4 ± 3.19A | 29.9 ± 2.75A |
| SW2 | 40 | 0.58 ± 0.31B | 25.6 ± 2.41B | 19.7 ± 1.73AB | 20.5 ± 3.98A | 5.05 ± 1.30A | 28.7 ± 4.18B | 45.8 ± 3.05B | 26.1 ± 2.46B |
| SW3 | 40 | 0.49 ± 0.28C | 25.1 ± 1.79B | 19.9 ± 1.57AB | 16.3 ± 1.96C | 4.07 ± 0.92B | 34.2 ± 3.70A | 45.4 ± 2.48B | 25.5 ± 1.88B |
| SW4 | 40 | 0.41 ± 0.15D | 25.4 ± 1.61B | 19.5 ± 1.73B | 15.6 ± 2.00C | 3.73 ± 0.82C | 35.4 ± 3.40A | 45.2 ± 2.48B | 25.8 ± 1.61B |
| CP0 | 80 | 0.50 ± 0.21 | 25.7 ± 2.83 | 19.7 ± 1.73 | 18.5 ± 3.71 | 3.92 ± 1.32 | 31.7 ± 5.41 | 45.9 ± 3.37 | 26.2 ± 2.94 |
| CP1 | 80 | 0.58 ± 0.29* | 26.9 ± 2.56* | 20.1 ± 1.71 | 16.5 ± 2.05* | 4.27 ± 0.82* | 31.7 ± 4.04 | 47.5 ± 3.47* | 27.5 ± 2.56* |
Intra-group differences in Cd fractionation and Cd availability resulting from FT, SW and CP
As shown in Table 1, there was a significant difference between each two treatments regarding the Wat-Cd content with FT frequency increasing, and its maximum emerged at F1. The maximal Exc-Cd content was at F9, much higher than at F0 and F1. Although both the Car-Cd and the Oxi-Cd contents showed a descending trend, no significant changes in the Car-Cd content were observed after F1. The maximal Org-Cd content was at F6, significantly higher than at other frequencies. The Res-Cd content related to F1 and F3 was markedly higher than that at F0 and F9 in spite that an uptrend was observed in the Res-Cd content. The M value was significantly higher at F9 than at F0.Variations in Cd fractions in soil were observed due to the different SW contents. Interestingly, an increase in the SW content caused a notable decrease in the Wat-Cd content. The maximal Exc-Cd content at SW1 was significantly higher than that under other SW content conditions. The Car-Cd content was markedly higher at SW1 than at SW4. The Oxi-Cd content at SW2 was the highest among the four-level of SW contents. A significant difference was observed in the Org-Cd content under various SW treatments. In detail, the minimum and the maximum was at SW1 and SW2, respectively. The Res-Cd content increased with the increase of the SW content. The K and M values were higher at SW1 than at other SW contents. The addition of CP caused a significant increase in the content of Wat-Cd, Exc-Cd, and Org-Cd, but a notable decrease in the Oxi-Cd content. In particular, the K and M values strongly increased due to the addition of CP, while no visible changes in the content of Car-Cd or Res-Cd was detected.
Inter-group differences in Cd fractionation and Cd availability among FT, SW and CP
The data presented in Table 2 demonstrated that each affecting factor (i.e., FT, SW, and CP) had significant effect on Cd fractionation and Cd availability. The relatively low eta value (<0.14) indicated a moderate effect of FT on the Car-Cd content, which also was observed for the effect of CP on the Exc-Cd content and Cd availability. No significant effects of SW on the Car-Cd content and of CP on the content of Car-Cd and Res-Cd were observed.| Source | Wat-Cd | Exc-Cd | Car-Cd | Oxi-Cd | Org-Cd | Res-Cd | K | M | |
|---|---|---|---|---|---|---|---|---|---|
| a The partial eta squared values were computed using alpha = 0.05, when P < 0.05, there was a significant difference, P = 0 implied P was below 0.001. | |||||||||
| SW | F | 314 | 42.0 | 2.65 | 77.5 | 130 | 64.0 | 31.4 | 45.9 |
| P | 0 | 0 | 0.05 | 0 | 0 | 0 | 0 | 0 | |
| etaa | 0.89 | 0.51 | 0.06 | 0.66 | 0.77 | 0.62 | 0.44 | 0.54 | |
| CP | F | 181 | 16.3 | 1.74 | 71.2 | 34.5 | 0 | 14.4 | 18.6 |
| P | 0 | 0 | 0.19 | 0 | 0 | 0.97 | 0 | 0 | |
| eta | 0.60 | 0.12 | 0.01 | 0.37 | 0.22 | 0 | 0.11 | 0.13 | |
| FT | F | 314 | 5.95 | 3.44 | 16.9 | 105 | 8.03 | 1.56 | 5.47 |
| P | 0 | 0 | 0.01 | 0 | 0 | 0 | 0.19 | 0 | |
| eta | 0.91 | 0.17 | 0.10 | 0.36 | 0.78 | 0.21 | 0.05 | 0.15 | |
| SW × CPe | F | 304 | 0.68 | 0.18 | 43.7 | 85.6 | 19.8 | 0.19 | 0.77 |
| P | 0 | 0.57 | 0.91 | 0 | 0 | 0 | 0.90 | 0.51 | |
| eta | 0.88 | 0.02 | 0 | 0.52 | 0.68 | 0.33 | 0.01 | 0.02 | |
| SW × FT | F | 38.7 | 2.12 | 0.33 | 1.45 | 12.9 | 1.02 | 1.02 | 2.36 |
| P | 0 | 0.02 | 0.98 | 0.15 | 0 | 0.44 | 0.44 | 0.01 | |
| eta | 0.80 | 0.18 | 0.03 | 0.13 | 0.56 | 0.09 | 0.09 | 0.19 | |
| CP × FT | F | 432 | 1.52 | 0.33 | 6.43 | 5.60 | 4.29 | 1.09 | 2.13 |
| P | 0 | 0.20 | 0.86 | 0 | 0 | 0 | 0.37 | 0.08 | |
| eta | 0.94 | 0.05 | 0.01 | 0.18 | 0.16 | 0.13 | 0.04 | 0.07 | |
| SW × CP × FT | F | 60.8 | 1.32 | 0.55 | 1.12 | 0.96 | 0.51 | 0.35 | 1.13 |
| P | 0 | 0.22 | 0.88 | 0.35 | 0.49 | 0.90 | 0.98 | 0.34 | |
| Eta | 0.86 | 0.12 | 0.05 | 0.10 | 0.09 | 0.05 | 0.03 | 0.10 | |
The binary interactions weakened the effects of FT and SW on the soil Cd fractionation and Cd availability. However, they enhanced the effects of CP on Cd fractionation except for Exc-Cd. The ternary interactions further weakened the binary interactions, while CP enhanced the binary interaction between SW and FT (SW × FT). The distribution showed a significant variation in the content of Wat-Cd, Exc-Cd and Org-Cd in soil as a result of SW × FT. The fractions of Cd except for Exc-Cd and Car-Cd in soil were remarkably affected by the binary interaction between CP and SW/FT (SW × CP and CP × FT). The ternary interaction of SW × CP × FT weakened the binary interactions on the Wat-Cd content. The M values were significantly affected only by SW × FT (Fig. 5).
Contribution rate of FT, SW and CP on Cd fractionation and Cd availability in soil
According to the results of the full factorial design, there was a significant effect (P < 0.05) on Cd fractionation and Cd availability resulting from the main and interactive effect of FT, SW and CP. In detail, the binary interactions had a significant effect (P < 0.05) on the content of Wat-Cd, Oxi-Cd, Org-Cd and Res-Cd. No significant ternary interactions (P > 0.05) were observed.Table 3 showed the estimated effects and percent distributions of each term. The percent contribution of the significant effects of SW, CP and FT was calculated based on the data presented in Table 3, which was displayed in Fig. 6. The main effect of SW significantly and positively (P < 0.05) influenced the Res-Cd content and its contribution was 79.0%, while it had a significant negative effect (P < 0.05) on the content of Wat-Cd, Exc-Cd, Car-Cd, Oxi-Cd, the K and the M values, with a contribution of 51.3%, 100%, 100%, 44.9%, 100% and 100%, respectively. The main effect of CP negatively influenced the Oxi-Cd content (P < 0.05), accounting for 34.0% of the total negative effect on the Oxi-Cd content. Nevertheless, it positively influenced (P < 0.05) the content of Exc-Cd, Org-Cd, the K and the M values, which accounted for 36.8%, 39.7%, 54.2% and 39.7% of the total positive effect on the corresponding variables, respectively.
| Source | Wat-Cd | Exc-Cd | Car-Cd | Oxi-Cd | Org-Cd | Res-Cd | K | M | |
|---|---|---|---|---|---|---|---|---|---|
| a E, computed using alpha = 0.05 via DOE, when P < 0.05, there was a significant difference; P = 0 implied P was below 0.001. T was the statistical value for evaluating the probability distribution, according to which P was obtained. | |||||||||
| SW | E | −0.26 | −3.69 | −0.80 | −2.98 | −0.15 | 7.87 | −4.75 | −3.94 |
| T | −5.4 | −7.65 | −2.18 | −5.06 | −0.66 | 9.87 | −7.52 | −8.08 | |
| P | 0 | 0 | 0.03 | 0 | 0.51 | 0 | 0 | 0 | |
| CP | E | 0.07 | 1.08 | 0.35 | −2.26 | 0.40 | 0.34 | 1.49 | 1.15 |
| T | 1.86 | 3.01 | 1.26 | −5.13 | 2.36 | 0.58 | 3.18 | 3.15 | |
| P | 0.06 | 0 | 0.21 | 0 | 0.02 | 0.56 | 0 | 0 | |
| FT | E | −0.11 | 1.86 | −0.48 | −0.59 | −0.61 | −0.06 | 1.26 | 1.74 |
| T | −2.43 | 3.89 | −1.31 | −1 | −2.69 | −0.07 | 2.02 | 3.6 | |
| P | 0.02 | 0 | 0.19 | 0.32 | 0.01 | 0.94 | 0.04 | 0 | |
| SW × CP | E | 0.14 | −0.33 | 0.20 | 1.06 | 0.61 | −1.70 | 0.01 | −0.19 |
| T | 2.97 | −0.69 | 0.55 | 1.81 | 2.68 | −2.13 | 0.02 | −0.39 | |
| P | 0 | 0.49 | 0.58 | 0.07 | 0.01 | 0.03 | 0.98 | 0.70 | |
| SW × FT | E | 0.03 | −0.64 | 0.49 | 0.39 | −0.16 | −0.12 | −0.11 | −0.60 |
| T | 0.54 | −0.99 | 1 | 0.49 | −0.52 | −0.11 | −0.13 | −0.93 | |
| P | 0.59 | 0.32 | 0.32 | 0.62 | 0.60 | 0.91 | 0.90 | 0.35 | |
| CP × FT | E | −0.13 | −0.82 | −0.11 | −1.40 | 0.38 | 2.09 | −1.06 | −0.95 |
| T | −2.77 | −1.72 | −0.30 | −2.4 | 1.65 | 2.64 | −1.69 | −1.97 | |
| P | 0.01 | 0.09 | 0.76 | 0.02 | 0.10 | 0.01 | 0.09 | 0.05 | |
| SW × CP × FT | E | −0.08 | 0.98 | −0.36 | −0.70 | 0.04 | 0.11 | 0.54 | 0.90 |
| T | −1.26 | 1.53 | −0.73 | −0.89 | 0.14 | 0.11 | 0.65 | 1.39 | |
| P | 0.21 | 0.13 | 0.47 | 0.37 | 0.89 | 0.91 | 0.52 | 0.17 | |
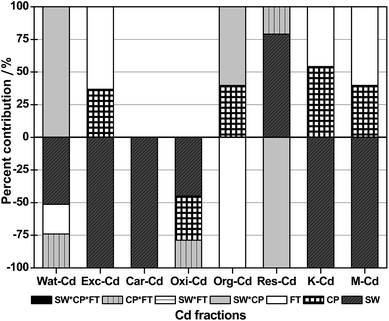 | ||
| Fig. 6 Contribution of main effects and interactions of FT, SW and CP on Cd fractionation and Cd availability in soil. | ||
Furthermore, the contribution generated from the binary interaction between SW and CP to the negative effect (P < 0.05) on the Res-Cd content was 100%, while its contribution to the positive effect (P < 0.05) on the content of Wat-Cd and Org-Cd was 100% and 60.3%, respectively. The binary interaction between CP and FT accounted for 21.0% of the positive effect (P < 0.05) on the Res-Cd content, and it caused a negative (P < 0.05) effect on the contents of Wat-Cd and Oxi-Cd, with a contribution of 25.9% and 21.1%, respectively. No other significant effects (P > 0.05) of the higher-order interaction on Cd fractionation and Cd availability were observed.
4. Discussion
Main and interactive effect of FT, SW and CP on Cd fractionation and Cd availability in soil
The FT process can occur with high levels of soil moisture and over a wide range of temperatures.23 Conversely, the FT process can alter the soil temperature, thermal and hydraulic conditions as well as water and energy exchanges at the land-atmosphere surface.29 These changing environmental factors can further influence Cd fractionation and Cd availability in soil.9 Thereby in this study, a reduction of the main effects on Cd fractionation in soil was observed when SW and FT interacted with each other. The main effect of CP on the K and M values indicated that the potential Cd availability moderately responded to the addition of CP. One possible reason is due to the fact that CP competes with Cd for sorption sites via electrostatic interactions and hydrogen bonding.30 On the other hand, CP can also affect Cd fractionation and availability via initial biotic transformation into chlorpyrifos-oxon.14 Chlorpyrifos-oxon can significantly alter the activity of soil organisms and enzymes.8 This can cause great changes in soil SOM and pH, which are key factors controlling Cd fractionation and availability.9 When CP interacted with FT or SW, the pesticide weakened the main effects of FT or SW, while the main effects of FT on the Wat-Cd content were enhanced. Simultaneously, the main effect of CP on the Cd fractionation in soil was altered when CP interacted with FT or SW. This phenomenon again suggested that natural environmental factors can alter the effects of chemicals due to changes in controlled conditions.5The results indicated that the binary interactions were further weakened by the ternary interactions. For example, SW × CP × FT further affected the binary interactions on the Wat-Cd content. It also suggested that natural environmental factors not only modify effects of chemicals directly but also influence them through interactions among them indirectly. This conclusion was also demonstrated in the previous study.2 One possible mechanism is the fact that CP inhibits the activity of soil enzymes or reduces the buffering capacity of soil,14 and thereby reduces the sensitivity of the potential Cd bioavailability in soil to FT and SW.
Acting mechanism of FT, SW and CP on Cd fractionation and Cd availability in soil
FT can alter soil surface area by destroying soil aggregates or by gathering fine particles around medium particles.31 In response, the number of soil sorption sites of H+ is increased or reduced, leading to changes in soil pH, which is a key factor dominating Cd behaviors in soil. Moreover, the soil aggregates destruction causes the release of Fe–Mn oxides and an increase in the DOM content. This potentially results in an increase of Cd availability/solubility in soil via the formation of soluble complexes.11,32 The decrease of soil pH caused by FT can also increase soil Cd availability/solubility. On this background, FT had a positive effect on the increase of labile Cd content, including Wat-Cd, Car-Cd, Oxi-Cd, and part of Org-Cd, as well as Cd availability. However, such increase may be inhibited by the elevated soil pH as function of FT. The above mechanism can also interpret the conclusion of this work that the contents of the different Cd fractions (especially the labile ones) varied with different FT frequencies.Moreover, the increased SW can enhance the extent to which soil aggregates are damaged by FT and thereby can promote the effects of FT on Cd fractionation and availability, which will decrease once SW exceeds the saturation value.12 Labile Cd fractions and Cd availability will decrease because the cationic Cd is more immobilized in the saturated soils with higher pH, lower Eh and potentially contain sulphides, compared to unsaturated soils.33 Cationic Cd dissolved in the soil solution can also migrate with soil water. As a function of FT, the unfrozen water with the dissolved Cd will migrate to the freeze front, beside the in situ water that is frozen during the freezing process.29 Moreover, an increase in temperature during the thawing process can be beneficial for ion exchange sorption but detrimental to specific sorption, which results in a process that is faster than the ion exchange sorption.34 Based on this information, FT can substantially affect the contents of Cd fractions. This is mainly due to the formation of chelates and precipitates on soil surface by combining with aluminosilicate and Fe–Mn oxides or by bonding with the oxygen or hydroxyl molecules within soil particles.35
Furthermore, FT can also affect Cd behavior via the alteration of the microbial structure and functions of soil, which readily respond to the soil humidity level and temperature.3,9 Simultaneously, the freezing process can cause a significant increase in the total amount of free amino acids and sugars, in combination with an increase in soil respiration and dehydrogenase activity.13 Nevertheless, either the microbial functions or the enzymatic activity can be easily affected by CP via ecological effects, organic effects and the coordination reaction.14 The complexation ability of the organic ligands that originate from CP can control the hysteresis quality of the Cd desorption process in soil, together with the soil colloid content.36,37 This is the reason that CP can alter the emerging time of visible changes in Cd fractions resulting from FT. The ability of CP that increased the potential Cd mobility coincided with the findings of other previous studies,32,38 where the presence of chloride and SOM promoted this process by forming soluble complexes.
Changes in Cd fractionation and Cd availability in soil caused by various treatments
In this study, the predominant Res-Cd content showed that most of the added Cd2+ could be adsorbed by the soil, whereas the relatively high Exc-Cd content suggested that Cd in the soil had a relatively strong potential bioavailability and mobility. These findings were consistent with those reported in other studies on the distribution of Cd in soil.39 Once added to soil, Wat-Cd is converted to relatively less soluble compounds within a very short period by inner-sphere surface complexation.40 Cd retention in the more persistent fractions over time results from reductions in the loosely bound fractions.41 Nevertheless, the transformation from Exc-Cd can be barricaded because Exc-Cd is likely hydrated by Cd2+ and adsorbed via outer-sphere surface complexation.42 The high K and M values observed in this study support this inference. Furthermore, the M values showed a variation similar to that of Exc-Cd. This phenomena could be explained by the higher content of Exc-Cd compared to the Wat-Cd content.5. Conclusions
The main effect of FT, SW and CP on Cd fractionation and availability was significant. The binary interactions weakened the main effect of FT or SW on Cd fractionation and availability, but enhanced the main effect of CP on Cd fractionation except for Exc-Cd. The ternary interactions further weakened the binary interactions, whereas CP enhanced the binary interaction between SW and FT. The binary interaction between SW and CP had a negative effect on Res-Cd (100%), but positively affected Wat-Cd and Org-Cd (100% and 60.3%). The bianry interaction between CP and FT had a positive effect on the Res-Cd content, but negatively affected Wat-Cd and Oxi-Cd (25.9% and 21.1%). No other significant effects of the higher-order interaction on Cd fractionation and Cd availability were observed. These results not only covered more innovative information to facilitate the identification of the multivariate interactions between natural and anthropogenic factors on Cd behavior in arable soil, but also showed a possibly new way to quantify the significant influence of multivariate factors was also given.Acknowledgements
This research benefited from financial support from the National Natural Science Foundation of China (Grant no. 41371018, 41271463), the “Twelfth Five-year Plan” for Sci & Tech Research of China (Grant no. 2012BAD15B05), and the GLOCOM project (Grant no. PIRSES-GA-2010-269233). Besides, the authors are very grateful for the assistance with data requirements provided by the Bawujiu Farm in Heilongjiang Province, China.References
- M. Amorim, C. Pereira, V. B. Menezes-Oliveira, B. Campos, A. Soares and S. Loureiro, Environ. Pollut., 2012, 160, 145–152 CrossRef CAS PubMed.
- R. Laskowski, A. J. Bednarska, P. E. Kramarz, S. Loureiro, V. Scheil, J. Kudłek and M. Holmstrup, Sci. Total Environ., 2010, 408(18), 3763–3774 CrossRef CAS PubMed.
- M. Broerse and C. A. van Gestel, Chemosphere, 2010, 79(9), 953–957 CrossRef CAS PubMed.
- M. J. Jonker, A. M. Piskiewick, N. I. Castellà and J. E. Kammenga, Environ. Toxicol. Chem., 2004, 23(6), 1529–1537 CrossRef CAS.
- M. Holmstrup, A. Bindesbøl, G. J. Oostingh, A. Duschl, V. Scheil, H. Köhler, S. Loureiro, A. M. Soares, A. L. Ferreira and C. Kienle, Sci. Total Environ., 2010, 408(18), 3746–3762 CrossRef CAS PubMed.
- F. Hao, S. Chen, W. Ouyang, Y. Shan and S. Qi, J. Hydrol., 2013, 486, 412–419 CrossRef PubMed.
- R. Reiser, M. Simmler, D. Portmann, L. Clucas, R. Schulin and B. Robinson, J. Environ. Qual., 2014, 43(3), 917–925 CrossRef PubMed.
- W. M. Williams, J. M. Giddings, J. Purdy, K. R. Solomon and J. P. Giesy, in Ecological risk assessment for chlorpyrifos in terrestrial and aquatic systems in the United States, Springer, 2014, vol. 231, pp. 77–117 Search PubMed.
- F. Wang, W. Ouyang, F. Hao, C. Lin and N. Song, J. Soils Sediments, 2014, 14(3), 451–461 CrossRef CAS.
- A. M. McIntyre and C. Guéguen, Chemosphere, 2013, 90(2), 620–626 CrossRef CAS PubMed.
- J. L. Campbell, A. B. Reinmann and P. H. Templer, Soil Sci. Soc. Am. J., 2014, 78(1), 297–308 CrossRef.
- E. van Bochove, D. Prévost and F. Pelletier, Soil Sci. Soc. Am. J., 2000, 64(5), 1638–1643 CrossRef CAS.
- K. C. Ivarson and F. J. Sowden, Can. J. Soil Sci., 1970, 50(2), 191–198 CrossRef CAS PubMed.
- J. P. K. Giesy, R. G. Solomon, C. Cutler, J. M. Giddings, D. Mackay, D. R. J. Moore, J. Purdy and W. M. Williams, in Ecological risk assessment for chlorpyrifos in terrestrial and aquatic systems in the United States, Springer, 2014, vol. 231, pp. 1–11 Search PubMed.
- I. H. Lee, Y. C. Kuan and J. M. Chern, J. Hazard. Mater., 2006, 138(3), 549–559 CrossRef CAS PubMed.
- F. J. Gravetter and L. B. Wallnau, in Essentials of statistics for the behavioral sciences, Cengage Learning, Belmont, U.S.A, 8th edn, 2013, vol. 1, p. 648 Search PubMed.
- M. Buragohain and C. Mahanta, Appl. Soft Comput., 2008, 8(1), 609–625 CrossRef PubMed.
- Y. Shan, M. Tysklind, F. Hao, W. Ouyang, S. Chen and C. Lin, J. Soils Sediments, 2013, 13(4), 720–729 CrossRef CAS.
- Y. S. Ok, A. R. Usman, S. S. Lee, S. A. Abd El-Azeem, B. Choi, Y. Hashimoto and J. E. Yang, Chemosphere, 2011, 85(4), 677–682 CrossRef CAS PubMed.
- W. Ouyang, H. Huang, F. Hao, Y. Shan and B. Guo, Sci. Total Environ., 2012, 432, 412–421 CrossRef CAS PubMed.
- I. Muhammad, M. Puschenreiter and W. W. Wenzel, Sci. Total Environ., 2012, 416, 490–500 CrossRef CAS PubMed.
- W. Ouyang, Y. Shan, F. Hao, S. Chen, X. Pu and M. K. Wang, Soil Tillage Res., 2013, 132, 30–38 CrossRef PubMed.
- H. A. Henry, Soil Biol. Biochem., 2007, 39(5), 977–986 CrossRef CAS PubMed.
- K. A. Yusuf, J. Agron., 2007, 6(2), 331 CrossRef CAS.
- S. Lee, H. Park, N. Koo, S. Hyun and A. Hwang, J. Hazard. Mater., 2011, 188(1), 44–51 CrossRef CAS PubMed.
- F. M. Abu-Zidan and H. O. Eid, Injury, 2015, 46(1), 136–141 CrossRef PubMed.
- P. Hammer, E. Richter, S. Rüsch-Gerdes, H. G. Walte, S. Matzen and C. Kiesner, J. Dairy Sci., 2015, 98(3), 1634–1639 CrossRef CAS PubMed.
- M. M. Tash and S. Alkahtani, Adv. Mater. Res., 2014, 881, 1317–1329 CrossRef.
- R. M. Nagare, R. A. Schincariol, W. L. Quinton and M. Hayashi, Hydrol. Earth Syst. Sci., 2012, 16, 501–515 CrossRef.
- X. Shen, W. Huang, C. Yao and S. Ying, Chemosphere, 2007, 67(10), 1927–1932 CrossRef CAS PubMed.
- E. Wang, R. M. Cruse, X. Chen and A. Daigh, Can. J. Soil Sci., 2012, 92(3), 529–536 CrossRef PubMed.
- L. Beesley, O. S. Inneh and G. J. Norton, Environ. Pollut., 2014, 186, 195–202 CrossRef CAS PubMed.
- E. Moreno-Jiménez, A. A. Meharg, E. Smolders, R. Manzano, D. Becerra, J. Sánchez-Llerena, Á. Albarrán and A. López-Piñero, Sci. Total Environ., 2014, 485–486, 468–473 CrossRef PubMed.
- H. K. Boparai, M. O. Joseph and D. M. Carroll, J. Hazard. Mater., 2011, 186(1), 458–465 CrossRef CAS PubMed.
- G. Mustafa, R. S. Kookana and B. Singh, Chemosphere, 2006, 64(5), 856–865 CrossRef CAS PubMed.
- R. L. Jefferies, N. A. Walker, K. A. Edwards and J. Dainty, Soil Biol. Biochem., 2010, 42(2), 129–135 CrossRef CAS PubMed.
- R. Naidu and R. D. Harter, Soil Sci. Soc. Am. J., 1998, 62(3), 644–650 CrossRef CAS.
- U. J. López-Chuken, U. López-Domínguez, R. Parra-Saldivar, E. Moreno-Jiménez, L. Hinojosa-Reyes, J. L. Guzmán-Mar and E. Olivares-Sáenz, Int. J. Environ. Sci. Technol., 2012, 9(1), 69–77 CrossRef PubMed.
- S. Lee, J. Lee, Y. Jeong Choi and J. Kim, Chemosphere, 2009, 77(8), 1069–1075 CrossRef CAS PubMed.
- R. Sikka and V. K. Nayyar, J. Res., 2011, 48(3–4), 112–118 Search PubMed.
- T. Lim, J. Tay and C. Teh, J. Environ. Qual., 2002, 31(3), 806–812 CrossRef CAS.
- A. Lu, S. Zhang and X. Shan, Geoderma, 2005, 125(3), 225–234 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |