A novel solid-state electrochemiluminescence sensor based on a Ru(bpy)32+/nano Sm2O3 modified carbon paste electrode for the determination of L-proline†
Morteza Hosseini*a,
Mohammad Reza Moghaddamb,
Farnoush Faridbodbc,
Parviz Norouzibc,
Mohammad Reza Karimi Purb and
Mohammad Reza Ganjalibc
aDepartment of Life Science Engineering, Faculty of New Sciences & Technologies, University of Tehran, Tehran, Iran. E-mail: smhosseini@khayam.ut.ac.ir
bCenter of Excellence in Electrochemistry, Faculty of Chemistry, University of Tehran, Tehran, Iran
cBiosensor Research Center, Endocrinology & Metabolism Molecular-Cellular Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran
First published on 21st July 2015
Abstract
A novel solid-state electrochemiluminescence (ECL) sensor was successfully developed for the determination of L-proline. The ECL signal was generated by a sensitive reaction between Ru(bpy)32+ and L-proline on a carbon paste electrode (CPE) modified with samarium oxide nanoparticles. The presence of Sm2O3 nanoparticles (NPs) in the carbon paste was found to enhance the electrochemiluminescence signal. Under optimized conditions, a linear relationship between the ECL intensity and the concentration of L-proline was observed in the linear range of 1.00 × 10−9 to 1.65 × 10−7 mol L−1 (R2 = 0.996) with a detection limit of 6.0 × 10−10 mol L−1 (S/N = 3) and a relative standard deviation of 3.3%. The method was sensitive, selective, simple and successfully applied to the analysis of L-proline in human serum and urine samples.
1. Introduction
Since the introduction of the electrochemiluminescence (ECL) technique based on tris(2,2′-bipyridyl) ruthenium(II) (Ru(bpy)32+) by Tokel and Bard,1 it has received considerable attention as a sensitive detection method for the chemical analysis of various analytes. The method has an excellent stability and high efficiency in aqueous media.2–9 Solution phase and solid-state methods are two different trends in generating ECL signals. Ru(bpy)32+ is one of the most important ECL probes, which is due to its regeneration properties during the ECL process, and that it is possible to immobilize it on electrode surfaces.10–13 Compared to the solution-phase ECL procedure, the immobilization of (Ru(bpy)32+) reduces the consumption of the costly reagent further by simplifying the experimental design. So far, several different methods have been used to immobilize (Ru(bpy)32+) on a solid surface, including the Langmuir–Blodgett,14 self-assembled,15 and electrostatic attachment techniques.16 However, the films produced through these techniques have proven to be unstable due to the need for the application of high potentials.17Carbon paste electrodes (CPEs) have been widely used in the determination of drugs, biomolecules, as well as other organic species owing to their easy preparation routes and wider potential window of −1.4 to +1.3 V (versus SCE). The residual currents associated with CPEs are also 10 times lower than those of glassy carbon or noble metal electrodes. The advantages of modified carbon paste electrodes (MCPEs) in electroanalytical chemistry have been well established. Hence, the development of CPE based devices for analytical applications is still of great interest.18
Currently, there is further interest in the development and application of new materials, like graphene, nanoparticles, and carbon nanotubes, capable of improving the surface properties of electrodes.19 The application of metal nanoparticles, among all, gives rise to distinct advantages such as higher mass transport, lower dependence on the solution resistance, low detection limit, and better signal-to-noise ratios.20,21
Considering their high mechanical stability, high dielectric constants, and large band gap characteristics, rare earth oxides have recently been extensively investigated for applications, including optoelectronic devices, switching mechanisms for logic devices, and memories.22 Rare earth oxides are well known to display a high surface basicity, a fast oxygen ion mobility and interesting catalytic properties.23
Samarium(III) oxide (Sm2O3, also known as Samaria), is one of the most important rare earth oxide materials and has been extensively studied due to its variable valence properties.24,25 Sm2O3 nanocrystals are promising building blocks for the bottom-up assembly of novel nanostructures with high potential applications in various fields such as solar cells,26 nanoelectronics,27 semiconductor gases and biochemical sensors.28
Recently, the analysis of L-proline has attracted considerable attention because of its important physical functions, broad distribution in nature and wide application in human life. Various analytical methods such as spectrophotometric,29 HPLC-amperometric,30 capillary electrophoresis–electrochemiluminescence31 and fluorescence32 methods have been used in the determination of amino acids for food stuff security and health purposes. Some of these methods are complex, time consuming and require tedious sample pretreatments or lack sufficient sensitivity, which limits their applications in many cases. Thus, developing new analytical methods for the sensitive and rapid detection of L-proline is still an attractive area of research. In this work, the development and application of a new modified Sm2O3 nanoparticle ECL carbon paste composite sensor for the determination of L-proline was investigated.
2. Experimental
2.1 Reagents and chemicals
All chemicals were of analytical reagent grade and used without further purification. Tris(2,2′-bipyridyl) ruthenium(II) (Ru(bpy)32+) chloride hexahydrate was obtained from Sigma Co., and used without further treatment. Nafion perfluorinated ion-exchange (5% solution in 90% light alcohol), carbon graphite powder and paraffin oil were obtained from Fluka (Buchs, Switzerland).2.2 Apparatus and procedure
A Perkin-Elmer LS50 luminescence spectrometer with a turn-off xenon lamp combined with a PalmSens PC potentiostat–galvanostat (Netherlands) and a three-electrode system was used for ECL spectrum measurement. Cyclic voltammetric (CV) studies were performed using a potentiostat–galvanostat with a conventional three-electrode set-up, in which the Sm2O3 NPs–Ru(bpy)32+–CPE, a Ag|AgCl|KCl(sat) electrode, and a platinum wire served as the working, reference and auxiliary electrodes, respectively. The electrochemical and ECL measurements were carried out in a 4 mL quartz cell, in which the working electrode was placed in an equatorial position in a quartz cell, while its surface precisely faced the window of a Fl-win lab photomultiplier LS 50 (Perkin-Elmer) and was fixed on a holder (see Fig. S1†). The detector and ECL cell were enclosed in a light tight black box. The spectral recording and cyclic voltammetry were conducted simultaneously with a spectral scan rate of 500 nm min−1 and a potential scan rate of 100 mV s−1 from 0 to 1.5 V.The size and morphology of the nanoparticles were measured by scanning electron microscopy (SEM) using a KYKY-EM 3200 Digital Scanning Electron Microscope (China).
2.3 Synthesis of samarium oxide nanoparticles
An aqueous samarium nitrate solution was prepared by dissolving samarium oxide powder in the appropriate amount of nitric acid by ultrasound. This solution was next diluted in ethanol and added to a 20 wt% polyvinyl alcohol–water solution under vigorous stirring in a glass beaker and the resulting mixture was heated up to 90 °C and stirred for 2 h. Upon condensation of the hydroxyl network (by giving off water), the gelation reaction was achieved leading to the formation of a dense porous gel, which was dried in a drying oven at 110 °C. The dried gel was then calcined at 400 °C for different periods of time to obtain samarium oxide NPs.33,342.4 Sensor preparation
The general procedure for the preparation of the CPE was as follows: prior to use, the graphite powder was treated at 800 °C for 60 s in a furnace and then cooled to room temperature in a desiccator in the presence of activated silica gel. 200 μL of 2.5 × 10−2 mol L−1 Ru(bpy)32+ was dispersed in a 500 μL 5 wt% Nafion solution to obtain a Ru(bpy)32+–Nafion solution. Graphite powder (90 mg) and the appropriate amount of the Sm2O3 nanoparticles were homogenized and thoroughly mixed with a 200 μL Ru(bpy)32+–Nafion solution. This solution was dried at room temperature for 30 min. The modified carbon powder and 50 μL of paraffin oil were then mixed together by hand-mixing in order to obtain a uniformly wetted paste. A portion of the modified carbon paste was placed at the end of a Pyrex tube (5 mm i.d.), and the electrical contact to the paste was established by inserting a copper wire down each tube and into the back of the mixture. Finally the modified CPE was smoothed using a fine piece of paper (see Fig. S2†).3. Results and discussion
3.1 Characterization of the Sm2O3 NPs
The morphology of the Sm2O3 nanoparticles and the incorporation of the Sm2O3 nanoparticles in the carbon paste samples were studied by SEM. The results shown in Fig. S3(A and B)† indicate that the produced nanoparticles are spherical in shape and have uniform surfaces with narrow size distribution.To obtain further information on the composite, energy-dispersive X-ray (EDX) analyses were performed on the samples without any treatments (Fig. S3C†). The EDX spectra proved the presence of samarium, oxygen, ruthenium and carbon as the main components of the nanocomposites. These data are summarized in the inset of Fig. S3C.†
3.2 Electrochemical and ECL behaviors of Ru(bpy)32+–Sm2O3 NPs–CPE
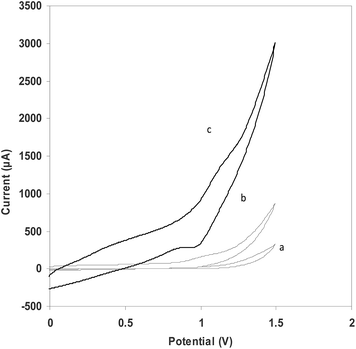
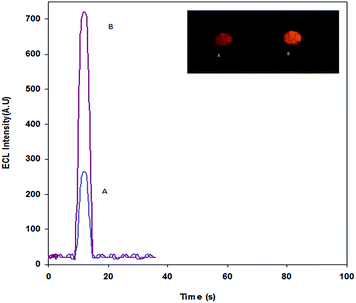
The catalytic effect of metal and metal oxide NPs such as Pt-NPs,35 Au-NPs,36 Ag-NPs,37 Pd-NPs38 and ZnO-NPs39 on chemiluminescence (CL) and ECL behavior have been shown in previous studies. In order to investigate the effect of the carbon paste modified Sm2O3 NPs on Ru(bpy)32+ oxidation and possible ECL reactions, CV was performed in the potential range between 0.0 and 1.5 V vs. Ag|AgCl|KCl(sat) at a scan rate of 100 mV s−1. Fig. 1 shows the cyclic voltammograms of the CPE and Ru(bpy)32+–Sm2O3 NPs–CPE in a pH 8.5 phosphate buffer at a scan rate of 100 mV s−1. The larger charging currents at the Sm2O3 NPs–Ru(bpy)32+–CPE observed in Fig. 1c are attributed to the increase in the electrode surface area due to the presence of Sm2O3 NPs. The Sm2O3 NPs–Ru(bpy)32+–CPE exhibited a couple of redox peaks at +1.153 V.Also, the immobilized Ru(bpy)32+ was revealed to be electroactive in the oxidation of L-proline. The result of the electrocatalytic process is a CL emitting species, which generates an ECL signal (Fig. 2).
In order to optimize the performance of the Sm2O3 NPs–Ru(bpy)32+–CPE ECL sensor towards L-proline detection, the effects of pH, Sm2O3 NPs loaded on the electrode surface, concentration of Ru(bpy)32+ and scan rate on the intensity of the ECL signal were investigated.
3.3 Effect of pH
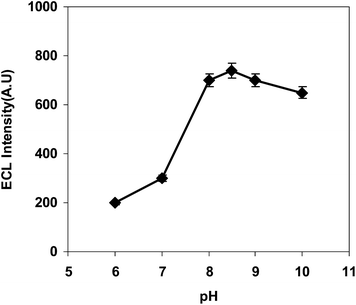
The effect of pH on the ECL intensity of L-proline was studied over a pH range from 6.0 to 10.0. Fig. 3 shows that the ECL intensity increased considerably with the rise in pH from 6.0 to 8.5. It was observed that at pH values higher than 8.5, the ECL intensity of L-proline decreased and hence pH 8.5 was chosen as the optimal pH value and a phosphate buffer solution at this pH value was used for ECL determinations.3.4 Effect of the Ru(bpy)32+ concentration
The effect of the concentration of Ru(bpy)32+ in the carbon paste electrode on the intensity of the ECL signal was also investigated, and the intensity of the ECL signal in the presence of 100 nM L-proline was found to linearly increase with increasing concentration of Ru(bpy)32+ from 1 × 10−2 mol L−1 to 2.5 × 10−2 mol L−1. Although further increase in the concentration of Ru(bpy)32+ (up to 2.5 × 10−2 mol L−1) did not cause further enhancement of the intensity of the ECL of the sensor, the background ECL signal of Ru(bpy)32+ steadily increased with increasing concentration of Ru(bpy)32+ from 1.0 × 10−2 mol L−1 to 2.5 × 10−2 mol L−1. Therefore, 2.5 × 10−2 mol L−1 was selected as the optimal concentration of Ru(bpy)32+ in the Sm2O3 NPs–Ru(bpy)32+–CPE used for the determination of L-proline.3.5 Effect of the Sm2O3 concentration
The effect of the loading of Sm2O3 on the intensity of the ECL signal was studied by casting different amounts of Sm2O3 nanoparticles in the carbon paste electrode. It is obvious from Fig. S4,† that the amount of Sm2O3 in the CPE is a crucial factor influencing the oxidation of Ru(bpy)32+ and finally the ECL response.Fig. S4† illustrates the effect of the amount of Sm2O3 on the intensity of the ECL signal of Ru(bpy)32+ in the presence of 100 nM L-proline. The ECL signal increased with increasing amount of Sm2O3 and reached a plateau at 5 mg of Sm2O3 in 90 mg of graphite. Thus, this ratio was used in the preparation of the carbon paste electrodes.
3.6 Effect of the scan rate
The effect of the scan rate (v) on the ECL and the CV signals of Ru(bpy)32+ was investigated in the presence of 100 nM L-proline. The results (Fig. 4a and b) indicated that, with increasing v, the intensity of the ECL signal reached a maximum at about 100 mV s−1. At the same time, with increasing scan rate from 50 to 350 mV s−1, both the oxidation and reduction currents increased linearly, while their peak potentials shifted in positive and negative directions and hence peak-to-peak separation increased. This indicates a surface controlled quasi-reversible electrode process. A scan rate of 100 mV s−1 was selected for further experiments, since the maximum ECL sensitivity was observed at this value.3.7 Interference studies
The influence of some common foreign species on the determination of L-proline was studied under the optimal experimental conditions stated above. The tolerable limit of a foreign species was taken as the concentration causing relative errors not greater than ± 5% in the ECL signal of L-proline. No interferences were found in the presence of up to 1000-fold (Mg2+, K+, Cl− and Na+), 500-fold L-tryptophan and L-serine, 300-fold (L-methionine, L-valine, L-lysine, L-asparagine), 200-fold uric acid, and 100-fold L-histidine, L-isoleucine, and L-leucine.3.8 Analytical performance
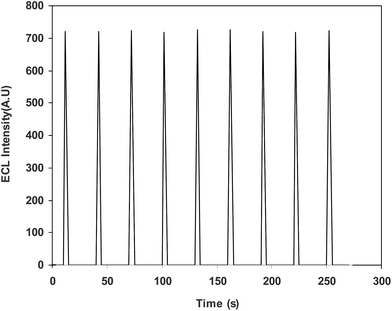
Under the optimal conditions given above, the response to L-proline was linear in the range from 1.0 × 10−9 to 1.65 × 10−7 mol L−1, with a regression equation of I = 5.0 × 109 c + 284, as well as a detection limit of 6.0 × 10−10 mol L−1 (S/N = 3). Fig. 5 shows the typical calibration traces recorded for L-proline using the proposed ECL sensor. The relative standard deviation was 3.3% for the determination of 1.0 × 10−8 mol L−1 L-proline (n = 7).The reproducibility of the signal of the modified electrode was also studied. Immersing the sensor in a phosphate buffer containing 100 nM L-proline led to no detectable change in the ECL intensity under repetitive cyclic potential scans, suggesting the good reproducibility of the ECL determination of L-proline (Fig. 6).
Also, the stability of the modified electrode was tested through the repetitive measurement of the ECL response. After two months, no evident decrease in the ECL response was observed and the sensor still maintained 92% of the original response. The results suggested that the modified electrode had a good stability.
A comparison between the results of this work and some other previously reported methods for the determination of L-proline is summarized in Table 1. In comparison with previous works, the proposed sensor has a wider linear range. The detection limit of the proposed sensors is 6.0 × 10−10 mol L−1 which is lower than spectrophotometric and fluorescence methods. In terms of the linear range and detection limit, it can be seen that the proposed sensor displays even more sensitivity than most of the reported methods.25–28
| Method | Detection limit (M) | Dynamic range (M) | Ref. |
|---|---|---|---|
| Spectrophotometry | 8.68 × 10−5 | 1.5 × 10−3 to 2.5 × 10−4 | 29 |
| HPLC-amperometric detector | 5.0 × 10−8 | 1.0 × 10−4 to 1.0 × 10−6 | 30 |
| Capillary electrophoresis–electrochemiluminescence | 2.0 × 10−6 | 2.0 × 10−3 to 8.0 × 10−6 | 31 |
| Fluorescence | 6.5 × 10−5 | 2.5 × 10−3 to 1.7 × 10−4 | 32 |
| Electrochemiluminescence | 6.0 × 10−10 | 1.0 × 10−9 to 1.65 × 10−7 | This work |
3.9 Mechanism of the enhancement of ECL by L-proline
The ECL signal of the Sm2O3 NPs–Ru(bpy)32+–CPE in the phosphate buffer in the absence of L-proline showed only a weak background ECL emission, indicating that the ECL emission of the system presumably arose from the energetic electron-transfer reaction between the electrogenerated Ru(bpy)33+ and the reducing intermediate, the deprotonated form of the oxidized L-proline ion free radical, to produce the excited state Ru(bpy)32+*, which is an emitting species. The electrochemical mechanism for the response was presumably analogous to that of the TPA–Ru(bpy)32+ system.40| Ru(bpy)32+ − e → Ru(bpy)33+ |
| L-Proline − e → L-proline˙+ |
| L-Proline˙+ → L-proline˙ + H+ |
| L-Proline˙ + Ru(bpy)33+ → L-proline fragments + Ru(bpy)32+* |
| Ru(bpy)32+* → Ru(bpy)32+ + hν |
3.10 Analytical applications
The applicability of the proposed sensor to the determination of L-proline in human serum and urine samples was examined. The ECL intensities after spiking a standard aliquot of L-proline to the diluted serum and urine solutions were obtained using the Sm2O3 NPs–Ru(bpy)32+–CPE at the optimal conditions described earlier. The concentrations were measured using the calibration plot, and the results are shown in Table 2. The recoveries indicate that both the accuracy and repeatability of the proposed sensor are very satisfactory. Based on the experimental results, this method has great potential for the determination of trace amounts of this compound in biological samples.4. Conclusions
In this work, an ECL sensor was fabricated for the determination of L-proline using carbon paste modified Sm2O3 nanoparticles. The immobilized Ru(bpy)32+ shows a diffusion electrode process and has an electrocatalytic action on the oxidation of L-proline, which results in the formation of an emitting species which produces the ECL signal. The proposed ECL sensor has a low detection limit of 6.0 × 10−10 mol L−1 and a linear range from 1.00 × 10−9 to 1.65 × 10−7 mol L−1. The applicability of the proposed sensor to the determination of L-proline in human samples was also evaluated, and it was found to have a good reproducibility and enough sensitivity for the detection of L-proline in different samples.Acknowledgements
The authors thank the research council of the University of Tehran for the financial support of this work.References
- N. E. Tokel and A. J. Bard, J. Am. Chem. Soc., 1972, 94, 2862 CrossRef CAS.
- M. M. Richter, Chem. Rev., 2004, 104, 3003 CrossRef CAS PubMed.
- J. Li and E. Wang, Chem. Rec., 2012, 1, 177 CrossRef PubMed.
- Y. Su and Y. Lv, RSC Adv., 2014, 4, 29324 RSC.
- Y. Xu, J. Liu, C. Gao and E. Wang, Electrochem. Commun., 2014, 48, 151 CrossRef CAS PubMed.
- O. Kargbo, S. N. Ding and L. Q. Li, Curr. Anal. Chem., 2014, 10, 622 CrossRef CAS.
- S. Deng and H. Ju, Analyst, 2013, 138, 43 RSC.
- M. Hosseini, N. Mirzanasiri, M. Rezapour, M. H. Sheikhha, F. Faridbod, P. Norouzi and M. R. Ganjali, Luminescence, 2015, 30, 376 CrossRef CAS PubMed.
- M. Hosseini, M. R. Karimipur, P. Norouzi, M. R. Moghaddam, F. Faridbod, M. R. Ganjali and J. Shamsi, Anal. Methods, 2015, 7, 1936 RSC.
- X. B. Yin, S. Dong and E. Wang, Trends Anal. Chem., 2004, 23, 432 CrossRef CAS.
- L. Ge, J. Yan, X. Song, M. Yan, S. Ge and J. Yu, Biomaterials, 2012, 33, 1024 CrossRef CAS PubMed.
- Z. Chen, Y. Liu, Y. Wang, X. Zhao and J. Li, Anal. Chem., 2013, 85, 4431 CrossRef CAS PubMed.
- G. A. Crespo, G. Mistlberger and E. Bakker, J. Am. Chem. Soc., 2012, 134, 205 CrossRef CAS PubMed.
- Z. Zhang and A. J. Bard, J. Phys. Chem., 1988, 92, 5566 CrossRef.
- Z. Guo, Y. Shen, M. Wang, F. Zhao and S. Dong, Anal. Chem., 2004, 76, 184 CrossRef CAS.
- Y. L. Zhou, Z. Li, N. F. Hu, Y. H. Zeng and J. T. Rusling, Langmuir, 2002, 18, 8537 Search PubMed.
- Y. S. Obeng and A. J. Bard, Langmuir, 1991, 7, 195 CrossRef CAS.
- I. Svancara, K. Vytras, K. Kalcher, A. Walcarius and J. Wang, Electroanalysis, 2009, 21, 7 CrossRef CAS PubMed.
- M. H. Parvin, Electrochem. Commun., 2011, 13, 366 CrossRef CAS PubMed.
- R. M. Penner and C. R. Martin, Anal. Chem., 1987, 59, 2625 CrossRef CAS.
- M. H. Parvin, M. B. Golivand, M. Najafi and S. M. Shariaty, J. Electroanal. Chem., 2012, 683, 31 CrossRef CAS PubMed.
- C. Constantinescu, V. Ion, A. C. Galca and M. Dinescu, Thin Solid Films, 2012, 520, 6393 CrossRef CAS PubMed.
- L. Eyring and K. A. Gschneider Jr., The Handbook on the Physics and chemistry of Rare Earths, North Holland, Amsterdam, 1979 Search PubMed.
- T. D. Nguyen, D. Mrabet and T. O. Do, J. Phys. Chem., 2008, 112, 15226 CAS.
- A. Rosengren and B. Johansson, Phys. Rev. B: Condens. Matter Mater. Phys., 1982, 26, 3068 CrossRef CAS.
- K. Kendall, Nature, 2000, 404, 233 CrossRef CAS PubMed.
- M. S. Gudiksen, L. J. Lauhon, J. Wang, D. C. Smith and C. M. Lieber, Nature, 2002, 415, 617 CrossRef CAS PubMed.
- P. W. Barone, S. Baik, D. A. Heller and M. S. Strano, Nat. Mater., 2005, 4, 86 CrossRef CAS PubMed.
- C. Truzzi, A. Annibaldi, S. Illuminati, C. Finale and G. Scarponi, Food Chem., 2014, 150, 477 CrossRef CAS PubMed.
- H. J. Sim, E. Moon, S. Y. Kim and S. P. J. Hong, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2013, 930, 70 CrossRef CAS PubMed.
- X. Jing, et al., Chin. J. Anal. Chem., 2005, 33, 785 Search PubMed.
- F. Robert-Peillard, J. L. Boudenne and B. Coulomb, Food Chem., 2014, 274 CrossRef CAS PubMed.
- M. R. Ganjali, H. Ganjali, M. Hosseini and P. Norouzi, Int. J. Electrochem., 2010, 5, 967 CAS.
- F. Faridbod, M. R. Ganjali, M. Hosseini and P. Norouzi, Int. J. Electrochem., 2012, 7, 1927 CAS.
- X. M. Chen, Z. J. Lin, Z. M. Cai, X. Chen, M. Oyama and X. R. Wang, J. Nanosci. Nanotechnol., 2009, 9, 2413 CrossRef CAS PubMed.
- B. Haghighi, S. Bozorgzadeh and L. Gorton, Sens. Actuators, B, 2011, 155, 577 CrossRef CAS PubMed.
- B. Haghighi and S. Bozorgzadeh, Microchem. J., 2010, 95, 192 CrossRef CAS PubMed.
- B. Haghighi and S. Bozorgzadeh, Anal. Chim. Acta, 2011, 697, 90 CrossRef CAS PubMed.
- B. Haghighi and S. Bozorgzadeh, Talanta, 2011, 85, 2189 CrossRef CAS PubMed.
- J. K. Leland and M. J. Powell, J. Electrochem. Soc., 1990, 137, 3127 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra06897e |
| This journal is © The Royal Society of Chemistry 2015 |