Hydrogen-bonded effects on supramolecular blue phase liquid crystal dimeric complexes†
Chong-Lun
Wei
a,
Te-Cheng
Chen
a,
Putikam
Raghunath
b,
Ming-Chang
Lin
b and
Hong-Cheu
Lin
*a
aDepartment of Materials Science and Engineering, National Chiao Tung University, Hsinchu, Taiwan, ROC. E-mail: linhc@mail.nctu.edu.tw
bCenter for Interdisciplinary Molecular Science, Department of Applied Chemistry, National Chiao Tung University, Hsinchu, Taiwan, ROC
First published on 12th June 2015
Abstract
In this study, a series of novel asymmetric hydrogen-bonded (H-bonded) dimeric complexes D/P and D/P* (proton donors D = A, A*, AF and AF*) were synthesized and self-assembled by appropriate molar ratios of H-donors (A, A*, AF and AF*) and H-acceptors (P and P*). In addition, the influences of the lateral fluoro-substituent of H-donors, the number (along with the position) of chiral centers and the molar ratio of H-donors and H-acceptors on the mesophasic behaviours (e.g., BPs) of asymmetric H-bonded dimeric complexes are investigated. Interestingly, the blue phase (i.e., BPI) was observed in complexes A*/P, A*/P*, AF*/P and AF*/P* containing at least a chiral center in H-donors (A* and AF*), including the widest BPI ranges of complexes AF*/P* (ca. 6 °C and 13 °C for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3
1 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol, respectively). For the first time, the hydrogen-bonded effects on supramolecular blue phase LCs are compared with their analogous covalent diads. Based on our theoretical calculation, we discovered that the bend angle plays an important role in manipulating the existence of the blue phase, which is preferred to appear at the bend angle within 132.1–152.9°. Hence, owing to inappropriate bend angles, both H-bonded dimeric complex A/P* and covalent diad A*-P (with bend angles of 162.0° and 126.5°, respectively) did not possess any blue phase.
1 mol, respectively). For the first time, the hydrogen-bonded effects on supramolecular blue phase LCs are compared with their analogous covalent diads. Based on our theoretical calculation, we discovered that the bend angle plays an important role in manipulating the existence of the blue phase, which is preferred to appear at the bend angle within 132.1–152.9°. Hence, owing to inappropriate bend angles, both H-bonded dimeric complex A/P* and covalent diad A*-P (with bend angles of 162.0° and 126.5°, respectively) did not possess any blue phase.
Introduction
The blue phase (BP) was discovered by Reinitzer and Lehmann in 1888, where an unusual blue light scattering phenomenon was quickly observed upon cooling from the isotropic phase (at the transition to the chiral nematic phase) in cholesteryl benzoate.1 These days, blue phase liquid crystals (BPLCs) have aroused attention in liquid crystal (LC) science due to their unique optical and electro-optical behaviors,2 such as selective reflections of circularly polarized light without birefringence. As alluded to above, BPLCs have several advantages:3 including fast response time (about sub-millisecond), no alignment layer required,4 and no typical birefringence. Therefore, BPLCs are potential materials to be used in liquid crystal displays (LCDs) and fast responsive modulators.5–8 However, BPs exhibited at very narrow temperature ranges (usually about 1 K) and appeared at high temperatures,9 which were usually observed between the isotropic phase and chiral nematic (N*) phase upon cooling. As reported, BPLCs were aggregated into internal helical alignments as “double twisted cylinders (DTC)” with three types of packing structures,10,11 which are categorized into BPI, BPII, and BPIII as follows: BPI is a body-center cubic structure, BPII is a simple cubic structure12,13 and BPIII is the same symmetry as the isotropic phase with an arbitrary orientation.14,15 So far, many strategies have been introduced to enlarge the BP temperature range and shift the BPs toward the room temperature.16 Kikuchi et al. have reported that they used polymers to stabilize the disclination of cubic lattice in BPs and extend the temperature range of BPs over 60 °C.17 Pivnenko's group developed novel dimeric BP materials with two symmetric liquid crystal mesogens, which have the BP phase temperature range ca. 44 °C.18 In the meanwhile, Yoshizawa group introduced a chiral structure of binaphthyl to induce blue phase with the BP temperature range approximately 30 °C.19 In addition, they synthesized T-shaped BPLCs20 and utilized U-shaped oligomers to stabilize BPs successfully.21 Yang's group also used nano-particles to stabilize BPs and decrease the V–T hysteresis of BPLCs.22 Moreover, chiral materials were doped into biaxial nametic LCs to induce PBs and extend their BP temperature ranges.23 Furthermore, a bent-core oxadiazole-based liquid crystal has been reported to exhibit a blue phase with a wide range (ca. 30 °C).24More recently, H-bonds have aroused much attention in novel materials because of the flexible characteristic of H-bonds,25–27 and the idea of supermolecules bearing noncovalent bond segments with higher flexibility, i.e. hydrogen-bonds (H-bonds), was applied to induce BPs28,30,32 or stabilize BPs.29,31 In addition, hydrogen-bonded bent- and T-shaped dopants (without BPs) were also utilized to stabilize BPs in LC mixtures.29 Yang's group showed that two complimentary liquid crystal moieties self-assembled by hydrogen bonds to form BPLC complexes with a wide BP range ca. 23 °C,30 and they also used chiral hydrogen-bonded assemblies to induce and stabilize BPs successfully.31 Besides, Zhang's group used rod-shaped hydrogen-bonded supermolecules to induce BPs.32 However, no comparisons between analogous H-bonded and covalent structures on the mesophasic properties of BPs and the BP range have been developed so far.
In this study, a series of asymmetric H-bonded dimeric complexes were synthesized and self-assembled by different molar ratios of proton donors and acceptors. Interestingly, the blue phase (i.e., BPI) was only induced in supramolecular complexes containing a chiral center in H-donors. For the first time, the hydrogen-bonded effects on supramolecular blue phase LCs are compared with their analogous covalent diads.33 In addition, the influences of the position of the chiral center (on H-donor and/or H-acceptor) and the lateral fluoro substituent on the BP temperature range were investigated systematically.
Experimental
Chemical analysis
1H NMR spectra were recorded on a Bruker Unity 300 MHz spectrometer using DMSO-d6, CDCl3 and THF-d8 as solvents. Elemental analyses (EA) were performed on a Heraeus CHN-OS RAPID elemental analyzer.Molecular simulation method
To gain insight into the electronic structures of eight asymmetric H-bonded dimeric complexes D/P and D/P* (where D = A, A*, AF and AF*) complexes, we have carried out density functional theory (DFT) calculations by using the Gaussian09 software package.34 Geometry optimization of all the ground state hydrogen-bonded structures was done by B97D Grimme's functional including dispersion corrections35 using the standard 6-31G(d,p) basis set. It was found that the performance of B97D method is remarkably good and reaching average on the CCSD(T) accuracy for noncovalently bound systems including many pure van der Waals complexes.35 The obtained minima are further confirmed by vibrational frequency calculations at the same level; only the lowest energy conformation is reported here. The electrostatic surface potential (ESP) was calculated using the Merz–Singh–Kollman (MK) scheme36 at the B97D/6-31G(d,p) level of theory.Liquid-crystalline and physical properties
Mesophasic textures of eight final asymmetric H-bonded dimeric complexes were characterized by polarizing optical microscopy (POM) using a Leica DMLP equipped with a temperature control hot stage (Mettler Toledo FP82HT). Temperatures and enthalpies of phase transitions were determined by differential scanning calorimetry (DSC, model: Perkin Elmer Pyris 7) under N2 at a heating and cooling rate of 1 °C min−1. Infrared (IR) spectra were investigated by Perk-Elmer Spectrum 100 instrument. Synchrotron powder X-ray diffraction (XRD) measurements were performed at beam-line BL17A equipped with magnetic of the National Synchrotron Radiation Research Center (NSRRC), Taiwan, where the wavelength of X-ray was 1.33336 Å. The powder samples were packed in a capillary tube and heated by a heat gun, for which the temperature controller is programmable by a PC with a PID feedback system. The scattering angle theta was calibrated by a mixture of silver behenate and silicon. Helical twisting power (HTP) measurements of H-bonded dimeric complexes and analogous covalent diads were preformed using Cano wedge cells (see Tables 3 and S2†); the commercial LC E7 (Merck) were used as a host.Preparation of materials
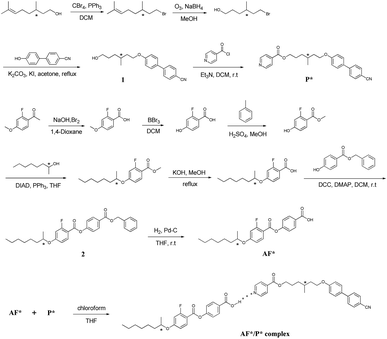
The synthesis of H-bonded dimeric complex AF*/P* was shown in Scheme 1.Preparation of samples
All asymmetric H-bonded dimeric complexes were constructed by mixing appropriate molar ratios of H-donors (A, A*, AF and AF*) and H-acceptors (P and P*) in solutions of chloroform/THF (∼2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vol), which were self-assembled into supramolecules by evaporating solvents slowly. All eight compounds of H-bonded in asymmetric heterodimers were formed D/P and D/P* (where D = A, A*, AF and AF*) complexes.
1 vol), which were self-assembled into supramolecules by evaporating solvents slowly. All eight compounds of H-bonded in asymmetric heterodimers were formed D/P and D/P* (where D = A, A*, AF and AF*) complexes.
Results and discussion
As shown in Fig. 1, a series of asymmetric H-bonded dimeric complexes D/N were synthesized and self-assembled by different molar ratios of proton donors (i.e., H-donors D = A, A*, AF and AF*) and proton acceptors (i.e., H-acceptors N = P and P*), where A, *, F and P denote acid, chiral, fluoro and pyridyl moieties, respectively. The hydrogen-bonded effects on supramolecular blue phase LCs of asymmetric H-bonded dimeric complexes D/P and D/P* are compared with their analogous covalent diads (see ref. 33), i.e., H-bonded dimeric complexes A*/P, A/P* and A*/P*vs. covalent diads A*-P, A-P* and A*-P*, respectively.33 | ||
| Fig. 1 Molecular structures of (a) asymmetric H-bonded dimeric complexes and (b) their analogous covalent diads (see ref. 33). | ||
Chemical synthesis
As illustrated in Fig. 1, H-donors (A, A*, AF and AF*) and H-acceptors (P and P*) of the asymmetric H-bonded dimeric complexes were synthesized according to Schemes S1–S3 (see the ESI†). These compounds with high purities were fully characterized by 1H-NMR (see the ESI†).Characterization of H-bonded structures
To study the existence and stability of H-bonds in all asymmetric heterodimers D/P and D/P* (D = A, A*, AF and AF*) complexes can be characterized by FTIR spectroscopy with temperature-various. Taking the asymmetric heterodimers of AF*/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) at mole ratio 1
1 mol) at mole ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of H-donors (AF*) and H-acceptors (P*) for example and its constituents AF* (H-donor) and P* (H-acceptor) are compared in Fig. 2(a).
1 of H-donors (AF*) and H-acceptors (P*) for example and its constituents AF* (H-donor) and P* (H-acceptor) are compared in Fig. 2(a).
 | ||
Fig. 2 IR spectra show (a) H-donor AF*, H-acceptor P* and asymmetric H-bonded dimeric complex AF*/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol). (b) AF*/P* (1 1 mol). (b) AF*/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) complex with variable temperatures (°C). 1 mol) complex with variable temperatures (°C). | ||
At room temperature, the O–H bands of pure AF* (H-donor) centered at about 2560 cm−1 and 2673 cm−1, when AF* (H-donor) and P* (H-acceptor) formed H-bonds these characteristic O–H bands disappear and two new broad O–H bands centered at 1920 cm−1 and 2500 cm−1 were observed which indicated that strong H-bonding formed between the pyridyl and the carboxylic acid groups in the asymmetric heterodimers.37,38 On the other hand, carboxylic acid groups C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration appeared at 1700 cm−1 and ester carbonyl groups C
O stretching vibration appeared at 1700 cm−1 and ester carbonyl groups C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration appeared at 1726 cm−1. In the asymmetric heterodimers of AF*/P* (1
O stretching vibration appeared at 1726 cm−1. In the asymmetric heterodimers of AF*/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol), a shoulder can be observed in the main peak located at 1726 cm−1. This shoulder is the carbonyl group which is in a less associated state than the pure AF* (H-donor) with a weaker carboxylic acid groups C
1 mol), a shoulder can be observed in the main peak located at 1726 cm−1. This shoulder is the carbonyl group which is in a less associated state than the pure AF* (H-donor) with a weaker carboxylic acid groups C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration appeared at 1700 cm−1.39–41 This is attributed to a carboxylic acid groups C
O stretching vibration appeared at 1700 cm−1.39–41 This is attributed to a carboxylic acid groups C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration at 1700 cm−1 in pure H-donor AF* which shifts toward higher wavenumber and overlaps with the band of the ester carbonyl groups at 1726 cm−1 in the asymmetric heterodimers of AF*/P* (1
O stretching vibration at 1700 cm−1 in pure H-donor AF* which shifts toward higher wavenumber and overlaps with the band of the ester carbonyl groups at 1726 cm−1 in the asymmetric heterodimers of AF*/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol). As cooling from the isotropic state to the blue phase I at 68 °C, two broad O–H bands centered at 1920 cm−1 and 2500 cm−1 still persist (see Fig. 2) due to the stable H-bonds. These consequences show that hydrogen bonds were formed between AF* (H-donor) and P* (H-acceptor) as well as other H-bonds asymmetric heterodimers.
1 mol). As cooling from the isotropic state to the blue phase I at 68 °C, two broad O–H bands centered at 1920 cm−1 and 2500 cm−1 still persist (see Fig. 2) due to the stable H-bonds. These consequences show that hydrogen bonds were formed between AF* (H-donor) and P* (H-acceptor) as well as other H-bonds asymmetric heterodimers.
Mesophasic and thermal properties
As shown in Tables 1 and 2, the phase transition temperatures, enthalpies and mesophasic ranges of all asymmetric H-bonded dimeric complexes were characterized by polarizer optical microscope (POM) and differential scanning calorimetry (DSC). The transition temperatures of BPs-chiral nematic (N*) were determined by POM (at a cooling rate of 0.5 °C min−1) due to their undetectable enthalpy changes by DSC. As the H-bonded complexes were self-assembled by two complimentary components of H-donors (A, A*, AF and AF*) and H-acceptors (P and P*), which formed –OH⋯N with a stronger tendency in contrast to –OH⋯C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O– of self-dimeric H-donors.42–44 However, while H-donors (A, A*, AF and AF*) were added over the stoichiometric amount (50 mol%) of two complimentary components (i.e., up to 66.7, 75 and 80 mol%), the over-supplied H-donors (>50 mol%) will generate self-dimers via the H-bonds of –OH⋯C
O– of self-dimeric H-donors.42–44 However, while H-donors (A, A*, AF and AF*) were added over the stoichiometric amount (50 mol%) of two complimentary components (i.e., up to 66.7, 75 and 80 mol%), the over-supplied H-donors (>50 mol%) will generate self-dimers via the H-bonds of –OH⋯C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O– after the production of supramolecular complexes. In order to comprehend the configurational influence of various asymmetric H-bonded dimeric complexes, including different numbers (or positions) of chiral centers and lateral fluoro substituent at the aromatic ring, on mesophasic and thermal properties of these eight asymmetric H-bonded dimeric complexes D/P and D/P* (where D = A, A*, AF and AF*) complexes are discussed as follows:
O– after the production of supramolecular complexes. In order to comprehend the configurational influence of various asymmetric H-bonded dimeric complexes, including different numbers (or positions) of chiral centers and lateral fluoro substituent at the aromatic ring, on mesophasic and thermal properties of these eight asymmetric H-bonded dimeric complexes D/P and D/P* (where D = A, A*, AF and AF*) complexes are discussed as follows:
| Complex | Molar ratio (H-donor vs. H-acceptor) | Phase transition temperatures (°C) [enthalpies (J g−1)] |
|---|---|---|
| a Peak temperatures in the DSC profiles obtained during the first heating and cooling cycles at a rate of 1 °C min−1. b Iso = isotropic phase; BPI = blue phase I; N = nematic phase; N* = chiral nematic phase; SmA = smectic A phase; Cr = crystal. Phase transition temperatures and enthalpies of complexes upon heating and those of pure components A, AF, P, and P* are shown in Tables S4 and S5, respectively. | ||
| A/P | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Iso 186.9 [4.91] N 154.9 [3.26] SmA 84.8 [3.01] Cr |
| AF/P | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Iso 168.7 [2.39] N 120.3 [4.77] SmA 74.9 [3.61] Cr |
| A/P* | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Iso 177.6 [3.78] N* 123.8 [4.16] SmA 70.1 [3.21] Cr |
| AF/P* | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Iso 157.5 [4.46] N* 82.1 [3.62] SmA 60.8 [3.14] Cr |
| Complex | Molar ratio (H-donor vs. H-acceptor) | Phase transition temperatures (°C) [enthalpies (J g−1)] | ΔTBP (°C) |
|---|---|---|---|
| a Peak temperatures in the DSC profiles obtained during the first heating and cooling cycles at a rate of 1 °C min−1. b Iso = isotropic phase; BPI = blue phase I; N* = chiral nematic phase; SmA = smectic A phase; Cr = crystal. c The transition to this phase was observed under the polarizing optical microscope and it was too weak to be recognized by the DSC. d The analogous covalent diads (see ref. 33). The phase transition temperatures and enthalpies of complexes upon heating and those of pure components A and AF* are shown in Tables S4 and S5, respectively. | |||
| A*/P | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Iso 150.3 [0.33] BPI 147.9cN* 126.3 [3.61] SmA 64.9 [1.32] Cr | 2.4 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Iso 133.1 [0.61] BPI 130.1cN* 73.5 [4.25] SmA 52.1 [1.03] Cr | 3.0 | |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Iso 106.8 [0.99] BPI 99.3cN* 93.9 [4.92] SmA 46.2 [2.11] Cr | 7.5 | |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Iso 150.1 [4.46] N* 80.5 [3.21] Cr | 0 | |
| A*/P* | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Iso 89.6 [0.41] BPI 84.9cN* 68.5 [3.41] SmA 41.5 [3.01] Cr | 4.7 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Iso 123.0 [0.43] BPI 116.1cN* 98.7 [2.88] SmA 44.7 [3.45] Cr | 6.9 | |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Iso 132.3 [0.63] BPI 124.1cN* 120.2 [5.69] SmA 72.6 [2.31] Cr | 8.2 | |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Iso 140.8 [3.23] N* 80.6 [1.23] Cr | 0 | |
| AF*/P | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Iso 96.9 [0.55] BPI 91.3cN* 73.5 [0.31] SmA 35.2 [0.98] Cr | 5.6 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Iso 106.7 [0.61] BPI 99.6cN* 95.1 [0.35] SmA 50.1 [0.54] Cr | 7.1 | |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Iso 120.9 [0.79] BPI 110.9cN* 92.4 [0.52] SmA 70.3 [0.72] Cr | 10.0 | |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Iso 126.1 [0.82] BPI 120.8cN* 72.6 [0.56] Cr | 5.3 | |
| AF*/P* | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Iso 70.3 [0.52] BPI 64.3cN* 42.3 [1.42] Cr | 6.0 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Iso 103.5 [0.62] BPI 95.0cN* 62.4 [0.95] Cr | 8.5 | |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Iso 109.2 [0.56] BPI 96.0cN* 61.8 [1.02] Cr | 13.2 | |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Iso 113.7 [0.58] BPI 107.4cN* 67.1 [1.11] Cr | 6.3 | |
| A*-P d | Iso 109.5 [11.76] N* 87.6 [5.43] SmA 79.7 [2.03] Cr | 0 | |
| A-P* d | Iso 182.2 [0.94] BPI 180.7cN* 106.2 [2.44] SmA 72.5 [1.22] Cr | 1.5 | |
| A*-P* d | Iso 108.0 [0.36] BPI 76.6cN* 51.9 [1.63] Cr | 31.4 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; D = A and AF) are demonstrated in Table 1. Without any chiral center on both H-donors and H-acceptors, H-bonded dimeric complexes A/P (1
1; D = A and AF) are demonstrated in Table 1. Without any chiral center on both H-donors and H-acceptors, H-bonded dimeric complexes A/P (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) and AF/P (1
1 mol) and AF/P (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) showed a phase sequence of Iso-nematic (N)-smectic A (SmA)-crystal. While H-acceptor P* was inserted a chiral center to the central linker which connected two different mesogenic units, complexes A/P* (1
1 mol) showed a phase sequence of Iso-nematic (N)-smectic A (SmA)-crystal. While H-acceptor P* was inserted a chiral center to the central linker which connected two different mesogenic units, complexes A/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) and AF/P* (1
1 mol) and AF/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) revealed a phase sequence of Iso-chiral nematic (N*)-SmA-crystal, which induced lower transition temperatures than A/P and AF/P, respectively. Due to the larger lateral fluoro substituent than hydrogen,45 complexes AF/P (1
1 mol) revealed a phase sequence of Iso-chiral nematic (N*)-SmA-crystal, which induced lower transition temperatures than A/P and AF/P, respectively. Due to the larger lateral fluoro substituent than hydrogen,45 complexes AF/P (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) and AF/P* (1
1 mol) and AF/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) with a lateral fluoro substituent illustrated that lower transition temperatures than A/P (1
1 mol) with a lateral fluoro substituent illustrated that lower transition temperatures than A/P (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) and A/P* (1
1 mol) and A/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol), respectively. The larger size occupation of both chiral center and lateral fluoro substituent would cause smaller π–π interactions of mesogens and thus to reduce the SmA phase range, i.e., so as to enlarge the N (or N*) phase range. Interestingly, comparing with the other similar chiral complexes of this study no blue phases were observed in both complexes D/P* (D = A and AF) due to their smaller biaxial ratios and larger bent angles, which will be explained by molecular modelling later.
1 mol), respectively. The larger size occupation of both chiral center and lateral fluoro substituent would cause smaller π–π interactions of mesogens and thus to reduce the SmA phase range, i.e., so as to enlarge the N (or N*) phase range. Interestingly, comparing with the other similar chiral complexes of this study no blue phases were observed in both complexes D/P* (D = A and AF) due to their smaller biaxial ratios and larger bent angles, which will be explained by molecular modelling later.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3
1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4
1 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; D = A* and AF*) are demonstrated in Table 2. A*/P, AF*/P and A*/P* exhibited the phase transition sequence of Iso-blue phase I (BPI)-N*-SmA-crystal rather than Iso-BPI-N*-crystal in AF*/P*, where the SmA phase was totally vanished in AF*/P* because of its largest size occupation of two chiral centers and one lateral fluoro substituent to eliminate the smectic layer structure. Surprisingly, all complexes D/P and D/P* (molar ratio = 1
1; D = A* and AF*) are demonstrated in Table 2. A*/P, AF*/P and A*/P* exhibited the phase transition sequence of Iso-blue phase I (BPI)-N*-SmA-crystal rather than Iso-BPI-N*-crystal in AF*/P*, where the SmA phase was totally vanished in AF*/P* because of its largest size occupation of two chiral centers and one lateral fluoro substituent to eliminate the smectic layer structure. Surprisingly, all complexes D/P and D/P* (molar ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, D = A* and AF* with a chiral center) revealed the blue phase (BPI), which is totally different from the previous complexes without a chiral center in H-donors. As shown in Table 2, while H-donors (A* and AF*) were added over the stoichiometric amount (50 mol%) of two complimentary components (i.e., up to 66.7 and 75 mol%), in which the excessive acids (A* and AF*) formed H-bonded dimers, the temperature range of BPI was extended at first. However, as H-donors (A* and AF*) were increased to 80 mol%, the BP temperature range was reduced or even disappeared in complexes A*/P (4
1, D = A* and AF* with a chiral center) revealed the blue phase (BPI), which is totally different from the previous complexes without a chiral center in H-donors. As shown in Table 2, while H-donors (A* and AF*) were added over the stoichiometric amount (50 mol%) of two complimentary components (i.e., up to 66.7 and 75 mol%), in which the excessive acids (A* and AF*) formed H-bonded dimers, the temperature range of BPI was extended at first. However, as H-donors (A* and AF*) were increased to 80 mol%, the BP temperature range was reduced or even disappeared in complexes A*/P (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) and A*/P* (4
1 mol) and A*/P* (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol), which might be attributed to the over-supply of A* without biaxiality and thus to eliminate the BP and induce the chiral nematic phase (N*) of pure A* only. Generally, the widest temperature ranges of BPI were observed at the molar ratio of 3
1 mol), which might be attributed to the over-supply of A* without biaxiality and thus to eliminate the BP and induce the chiral nematic phase (N*) of pure A* only. Generally, the widest temperature ranges of BPI were observed at the molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (75 mol%) in all D/P and D/P* (D = A* and AF* with a chiral center), where the equal molar ratio (1
1 (75 mol%) in all D/P and D/P* (D = A* and AF* with a chiral center), where the equal molar ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) of acid dimer D and supramolecular complexes D/P (or D/P*) were mixed. It is well known that the concentration of chiral dopant was increased and the BP temperature range would be extended.46 In fact, the over-supplied H-donors (>50 mol%) will generate self-dimers and the excessive acid dimers acted as chiral dopants and supplied extra helical twisting power to broaden the BPI temperature range. Oppositely, with over-supplied H-acceptors (P and P* > 50 mol%) the excessive H-acceptors (no mesophases shown in Table S5†) will not only damage the BP temperature range but also eliminate the occurence of mesophases (including BP), which have been confirmed by AF*/P* with 1
1 mol) of acid dimer D and supramolecular complexes D/P (or D/P*) were mixed. It is well known that the concentration of chiral dopant was increased and the BP temperature range would be extended.46 In fact, the over-supplied H-donors (>50 mol%) will generate self-dimers and the excessive acid dimers acted as chiral dopants and supplied extra helical twisting power to broaden the BPI temperature range. Oppositely, with over-supplied H-acceptors (P and P* > 50 mol%) the excessive H-acceptors (no mesophases shown in Table S5†) will not only damage the BP temperature range but also eliminate the occurence of mesophases (including BP), which have been confirmed by AF*/P* with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molar ratios. Among all complexes in Table 2, AF*/P* with 1
3 molar ratios. Among all complexes in Table 2, AF*/P* with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3
1 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratios have the broadest BPI temperature ranges of 6.0 °C and 13.2 °C, respectively, where the lateral fluoro substituent (with a stronger dipole moment) and double chiral centers eliminate the smectic layer structure owing to its larger steric hindrance. On the contrary, complexes A*/P (with various molar ratios) have the narrowest corresponding BPI temperature ranges. In addition, with various molar ratios, complexes AF*/P have larger BPI temperature ranges than A*/P*, which suggests that the lateral fluoro substitution (in H-donor) is more favourable to broaden the BPI range than double chiral centers in both H-donor and H-acceptor.
1 molar ratios have the broadest BPI temperature ranges of 6.0 °C and 13.2 °C, respectively, where the lateral fluoro substituent (with a stronger dipole moment) and double chiral centers eliminate the smectic layer structure owing to its larger steric hindrance. On the contrary, complexes A*/P (with various molar ratios) have the narrowest corresponding BPI temperature ranges. In addition, with various molar ratios, complexes AF*/P have larger BPI temperature ranges than A*/P*, which suggests that the lateral fluoro substitution (in H-donor) is more favourable to broaden the BPI range than double chiral centers in both H-donor and H-acceptor.
The hydrogen-bonded effects on supramolecular blue phase LCs are compared with their analogous covalent diads, i.e., H-bonded dimeric complexes A*/P, A/P* and A*/P*vs. covalent diads A*-P, A-P* and A*-P*, respectively. As illustrated in Table 2, the analogous covalent diad A*-P* with double chiral centers has a broader BPI temperature range than H-bonded dimeric complex A*/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol). However, H-bonded dimeric complex A*/P (1
1 mol). However, H-bonded dimeric complex A*/P (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) with a dipole moment of 6.0 D and a bent angle of 152.9° has a broader BPI temperature range than the analogous covalent diad A-P* with a dipole moment of 13.2 D and a bent angle of 141.1°. In general, H-bonded dimeric complexes possess smaller dipole moments (5.8–7.4 D) and larger bent angles (149.9–162.0°) in contrast to those (11.9–13.2 D and 126.5–141.1°, respectively) of their analogous covalent diads. However, the large variations of dipole moments in H-bonded dimeric complexes and their analogous covalent diads will not affect the presence of the blue phase. We found that the bent angle plays an important role to manipulate the existence of the blue phase, which is preferred to appear at the bent angle ranging 132.1–152.9°. Thus, due to lacks of proper bent angles within 132.1–152.9°, both H-bonded dimeric complex A/P* and covalent diad A*-P (with bent angles of 162.0° and 126.5°, respectively) did not possess any blue phase.
1 mol) with a dipole moment of 6.0 D and a bent angle of 152.9° has a broader BPI temperature range than the analogous covalent diad A-P* with a dipole moment of 13.2 D and a bent angle of 141.1°. In general, H-bonded dimeric complexes possess smaller dipole moments (5.8–7.4 D) and larger bent angles (149.9–162.0°) in contrast to those (11.9–13.2 D and 126.5–141.1°, respectively) of their analogous covalent diads. However, the large variations of dipole moments in H-bonded dimeric complexes and their analogous covalent diads will not affect the presence of the blue phase. We found that the bent angle plays an important role to manipulate the existence of the blue phase, which is preferred to appear at the bent angle ranging 132.1–152.9°. Thus, due to lacks of proper bent angles within 132.1–152.9°, both H-bonded dimeric complex A/P* and covalent diad A*-P (with bent angles of 162.0° and 126.5°, respectively) did not possess any blue phase.
Optical and XRD investigations
As shown in Fig. 3, a typical schlieren texture47 with four brushes at 145.3 °C in Fig. 3(a) and a fingerprint texture at 139.2 °C in Fig. 3(b) were observed in the asymmetric H-bonded dimeric complexes AF/P (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) and A/P* (1
1 mol) and A/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol), respectively. Regarding the mesophases of asymmetric H-bonded dimeric complexes AF*/P (3
1 mol), respectively. Regarding the mesophases of asymmetric H-bonded dimeric complexes AF*/P (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) and AF*/P* (3
1 mol) and AF*/P* (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol), we investigated the phase transition sequence by POM on the cooling process with a cooling rate of 0.5 °C min−1. Fig. 4(a–d) show the phase transition sequence of AF*/P (3
1 mol), we investigated the phase transition sequence by POM on the cooling process with a cooling rate of 0.5 °C min−1. Fig. 4(a–d) show the phase transition sequence of AF*/P (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol): the isotropic phase (Iso)-blue phase I (PBI) which has platelet textures with different colors and fine stripes-chiral nematic phase (N*)-homeotropic state of the Smectic A phase (the inset is the conoscopic pattern), respectively. Similarly, the phase transition sequence of AF*/P* (3
1 mol): the isotropic phase (Iso)-blue phase I (PBI) which has platelet textures with different colors and fine stripes-chiral nematic phase (N*)-homeotropic state of the Smectic A phase (the inset is the conoscopic pattern), respectively. Similarly, the phase transition sequence of AF*/P* (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) is shown in Fig. 5(a–c): Iso-BPI-N*, respectively.
1 mol) is shown in Fig. 5(a–c): Iso-BPI-N*, respectively.
All phase transition sequences were investigated by XRD measurements (see Fig. S1 and Table S1†). The sharp reflection peaks of XRD patterns (representing d-spacing layer) were observed in the small angle area of the smectic phase (including SmA), but only broad peaks were revealed in the wide angle area of the N and N* phases. As shown in Table S1 (see the ESI†), A/P (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol), A*/P (1
1 mol), A*/P (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol), AF/P (1
1 mol), AF/P (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) and AF*/P (1
1 mol) and AF*/P (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) processed d-spacing values of 48.1 Å, 47.5 Å, 47.8 Å and 46.7 Å, respectively. In addition, A/P* (1
1 mol) processed d-spacing values of 48.1 Å, 47.5 Å, 47.8 Å and 46.7 Å, respectively. In addition, A/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol), A*/P* (1
1 mol), A*/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) and AF/P* (1
1 mol) and AF/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) also exhibited d-spacing values of 48.5 Å, 47.7 Å and 49.1 Å, respectively. Their d-spacing values (d) were similar to their corresponding molecular lengths (L) by the theoretical simulation (shown below), which suggested the d-spacing values of the SmA phase in these diads are monolayer arrangements (i.e., d ∼ L). Therefore, the SmA phase was verified not only by the homeotropic texture of POM photo-images but also by the sharp peaks (d-spacing values) of XRD measurements.
1 mol) also exhibited d-spacing values of 48.5 Å, 47.7 Å and 49.1 Å, respectively. Their d-spacing values (d) were similar to their corresponding molecular lengths (L) by the theoretical simulation (shown below), which suggested the d-spacing values of the SmA phase in these diads are monolayer arrangements (i.e., d ∼ L). Therefore, the SmA phase was verified not only by the homeotropic texture of POM photo-images but also by the sharp peaks (d-spacing values) of XRD measurements.
Theoretical simulation
To substantiate the structural changes observed in the experiment for the eight asymmetric H-bonded dimeric complexes and to provide a better insight into the effects of the temperature range and stabilization on BPs (see Table 3 and the ESI†). We have a detailed quantum chemical calculation for the geometrical parameters of hydrogen bond lengths, molecular lengths, breadth, bent angles, dipole moments and charge density distribution properties. The electronic optimization for all asymmetric H-bonded dimeric complexes D/P and D/P* (D = A, A*, AF and AF*) in the gas-phase was carried out at the B97D/6-31G(d,p) level. Among all eight complexes, four structures of asymmetric H-bonded dimeric complexes are more efficient for the formation of BPs, including A*/P (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol), AF*/P (1
1 mol), AF*/P (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol), A*/P* (1
1 mol), A*/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) and AF*/P* (1
1 mol) and AF*/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol), bearing the chiral center (on H-donor and/or H-acceptor) and the lateral fluoro substituent. Their main geometrical parameters are summarized in Table 3 and structures are shown in Fig. 6. The geometrical properties of the remaining complexes A/P (1
1 mol), bearing the chiral center (on H-donor and/or H-acceptor) and the lateral fluoro substituent. Their main geometrical parameters are summarized in Table 3 and structures are shown in Fig. 6. The geometrical properties of the remaining complexes A/P (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol), AF/P (1
1 mol), AF/P (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol), A/P* (1
1 mol), A/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) and AF/P* (1
1 mol) and AF/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) are shown in Fig. S2 and Tables S2 and S3 of the ESI.† Because the biaxiality, bent angle and dipole moment of the molecule play a very important role in the widening and stabilizing the BP temperature range, these parameters are defined as follows: L (length along the long axis), biaxiality equals W1/W2 (W1: width along the short axis normal to the benzene plane and W2: width along the short axis parallel to the benzene plane) and bent angles are measured as the angles between the centers of the first, central and final benzene rings of the bent-core structures (as shown in Fig. 6). The calculated hydrogen bond lengths and hydrogen bond bent angles in complexes A*/P (1
1 mol) are shown in Fig. S2 and Tables S2 and S3 of the ESI.† Because the biaxiality, bent angle and dipole moment of the molecule play a very important role in the widening and stabilizing the BP temperature range, these parameters are defined as follows: L (length along the long axis), biaxiality equals W1/W2 (W1: width along the short axis normal to the benzene plane and W2: width along the short axis parallel to the benzene plane) and bent angles are measured as the angles between the centers of the first, central and final benzene rings of the bent-core structures (as shown in Fig. 6). The calculated hydrogen bond lengths and hydrogen bond bent angles in complexes A*/P (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol), AF*/P (1
1 mol), AF*/P (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol), A*/P* (1
1 mol), A*/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) and AF*/P* (1
1 mol) and AF*/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) are almost similar and ca. 1.7 Å and 178°, respectively.
1 mol) are almost similar and ca. 1.7 Å and 178°, respectively.
Complex (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) 1 mol) |
Length L(Å) | Breadth W1(Å) | Breadth W2 (Å) | Biaxial parameter (W1/W2) | H-bond length (Å) | H-bond bent angle (deg) | Bent anglea (deg) | Dipole moment (Debye, D) | HTPb (μm−1) |
|---|---|---|---|---|---|---|---|---|---|
| a Bent angle (°) measured as the angle between the first, central and final benzene rings' centers of the bent-core structures. The detailed dipole moments of the lowest energy structures are given (see Tables S2 and S3 of the ESI). b HTP values of H-bonded complexes and analogous covalent diads (see Table S2) were obtained by Cano wedge cells. | |||||||||
| A*/P | 46.7 | 9.5 | 4.9 | 1.94 | 1.7 | 178.3 | 152.9 | 6.0 | 2.56 |
| A*/P* | 46.5 | 9.3 | 6.0 | 1.55 | 1.7 | 178.8 | 150.6 | 6.6 | 2.78 |
| AF*/P | 46.7 | 9.6 | 4.9 | 1.96 | 1.7 | 178.5 | 152.5 | 6.7 | 2.50 |
| AF*/P* | 46.4 | 9.4 | 6.2 | 1.52 | 1.7 | 178.5 | 149.9 | 7.4 | 2.94 |
As shown in Table 3, the HTP values of four H-bonded dimeric complexes with BPs, including A*/P, AF*/P, A*/P* and AF*/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol), bearing the chiral center (on H-donor and/or H-acceptor) and the lateral fluoro substituent are listed and compared with their analogous covalent diads (including A*-P, A-P* and A*-P* in Table S2†). All H-bonded dimeric complexes A*/P (2.56 μm−1), A/P* (2.44 μm−1) and A*/P* (2.78 μm−1) have smaller HTP values than their analogous covalent diads A*-P (3.05 μm−1), A-P* (2.97 μm−1) and A*-P* (3.22 μm−1), respectively, where the lower HTP values of H-bonded dimeric complexes might be attributed to the higher flexibilities of their H-bonded structures. Moreover, the HTP values of H-bonded dimeric complexes have the trend of A*/P* > A*/P > A/P* similar to that of covalent diads A*-P* > A*-P > A-P*. Hence, the highest HTP values of A*/P* and A*-P* (with two chiral centers) along with the lowest HTP values of A/P* and A-P* (with a single chiral center on the middle of the flexible spacer) are obtained in both H-bonded complexes and covalent diads, respectively.
1 mol), bearing the chiral center (on H-donor and/or H-acceptor) and the lateral fluoro substituent are listed and compared with their analogous covalent diads (including A*-P, A-P* and A*-P* in Table S2†). All H-bonded dimeric complexes A*/P (2.56 μm−1), A/P* (2.44 μm−1) and A*/P* (2.78 μm−1) have smaller HTP values than their analogous covalent diads A*-P (3.05 μm−1), A-P* (2.97 μm−1) and A*-P* (3.22 μm−1), respectively, where the lower HTP values of H-bonded dimeric complexes might be attributed to the higher flexibilities of their H-bonded structures. Moreover, the HTP values of H-bonded dimeric complexes have the trend of A*/P* > A*/P > A/P* similar to that of covalent diads A*-P* > A*-P > A-P*. Hence, the highest HTP values of A*/P* and A*-P* (with two chiral centers) along with the lowest HTP values of A/P* and A-P* (with a single chiral center on the middle of the flexible spacer) are obtained in both H-bonded complexes and covalent diads, respectively.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The asymmetric H-bonded dimeric complexes AF*/P* (1
1. The asymmetric H-bonded dimeric complexes AF*/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) ∼6.0 °C and A*/P* (1
1 mol) ∼6.0 °C and A*/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) ∼4.7 °C are much wider than AF*/P (1
1 mol) ∼4.7 °C are much wider than AF*/P (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) ∼5.6 °C and A*/P (1
1 mol) ∼5.6 °C and A*/P (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) ∼2.4 °C, because complexes D/P* (D = A* and AF*) have one more chiral center on H-acceptor than complexes D/P (D = A* and AF*). We further investigate the deviations of biaxiality, dipole moment and bent angle between both complexes D/P* and D/P (D = A* and AF*). The results show that the performance of biaxiality for complexes A*/P (1
1 mol) ∼2.4 °C, because complexes D/P* (D = A* and AF*) have one more chiral center on H-acceptor than complexes D/P (D = A* and AF*). We further investigate the deviations of biaxiality, dipole moment and bent angle between both complexes D/P* and D/P (D = A* and AF*). The results show that the performance of biaxiality for complexes A*/P (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) and AF*/P (1
1 mol) and AF*/P (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) (biaxiality parameter ∼1.94 and ∼1.96, respectively) is greater than complexes A*/P* (1
1 mol) (biaxiality parameter ∼1.94 and ∼1.96, respectively) is greater than complexes A*/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) and AF*/P* (1
1 mol) and AF*/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) (biaxiality parameter ∼1.55 and ∼1.52, respectively). The bent angle and dipole moment results of A*/P* (1
1 mol) (biaxiality parameter ∼1.55 and ∼1.52, respectively). The bent angle and dipole moment results of A*/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) (bent angle ∼150.6° and dipole moment ∼6.6 D) and AF*/P* (1
1 mol) (bent angle ∼150.6° and dipole moment ∼6.6 D) and AF*/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) (bent angle ∼149.9° and dipole moment ∼7.4 D) indicate a greater bent shape and larger dipole moment value than A*/P (1
1 mol) (bent angle ∼149.9° and dipole moment ∼7.4 D) indicate a greater bent shape and larger dipole moment value than A*/P (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) (bent angle ∼152.9° and dipole moment ∼6.0 D) and AF*/P (1
1 mol) (bent angle ∼152.9° and dipole moment ∼6.0 D) and AF*/P (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) (bent angle ∼152.5° and dipole moment ∼6.7 D), respectively. Hence, the chiral center is introduced to H-acceptor to increase the value of dipole moment and bent shape of molecule; thus it is more helpful to stabilize and extend the BP temperature range than biaxiality.
1 mol) (bent angle ∼152.5° and dipole moment ∼6.7 D), respectively. Hence, the chiral center is introduced to H-acceptor to increase the value of dipole moment and bent shape of molecule; thus it is more helpful to stabilize and extend the BP temperature range than biaxiality.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) ∼6.0 °C and AF*/P (1
1 mol) ∼6.0 °C and AF*/P (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) ∼5.6 °C are much wider than A*/P* (1
1 mol) ∼5.6 °C are much wider than A*/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) ∼4.7 °C and A*/P (1
1 mol) ∼4.7 °C and A*/P (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) ∼2.4 °C complexes. Our theoretical results show that the molecular bent angles and dipole moment values of lateral fluoro substituent complexes AF*/P (1
1 mol) ∼2.4 °C complexes. Our theoretical results show that the molecular bent angles and dipole moment values of lateral fluoro substituent complexes AF*/P (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) and AF*/P* (1
1 mol) and AF*/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) are greater than those without fluorine substitution complexes A*/P (1
1 mol) are greater than those without fluorine substitution complexes A*/P (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) and A*/P* (1
1 mol) and A*/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) (See Table 3). As alluded to above, we concluded that the lateral fluoro substituent at H-donors delivered a high electronegativity and dipole moment, hence the fluorine is more favourable to broaden the BP range. Although the performance of biaxiality for complex AF*/P* (1
1 mol) (See Table 3). As alluded to above, we concluded that the lateral fluoro substituent at H-donors delivered a high electronegativity and dipole moment, hence the fluorine is more favourable to broaden the BP range. Although the performance of biaxiality for complex AF*/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) is only 1.52, complex AF*/P* (1
1 mol) is only 1.52, complex AF*/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) has a large value of dipole moment (∼7.4 D) and a bent shape of molecular geometry (∼149.9°). The results show that the ground state dipole moments for all complexes based on the Cartesian coordinate system are found to be oriented in XY-plane and mainly dominant on the X axis, lying on the backbones of the molecule (see ESI Table S3†). In this study, the molar ratio H-donors (AF*) vs. H-acceptors (P*) was 3
1 mol) has a large value of dipole moment (∼7.4 D) and a bent shape of molecular geometry (∼149.9°). The results show that the ground state dipole moments for all complexes based on the Cartesian coordinate system are found to be oriented in XY-plane and mainly dominant on the X axis, lying on the backbones of the molecule (see ESI Table S3†). In this study, the molar ratio H-donors (AF*) vs. H-acceptors (P*) was 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (75 mol%) which exhibited the widest temperature range of BP (∼13.2 °C). In addition to the analysis of the electron density distribution properties, we also generated the electrostatic potential of asymmetric H-bonded dimeric complexes A*/P (1
1 (75 mol%) which exhibited the widest temperature range of BP (∼13.2 °C). In addition to the analysis of the electron density distribution properties, we also generated the electrostatic potential of asymmetric H-bonded dimeric complexes A*/P (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol), AF*/P (1
1 mol), AF*/P (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol), A*/P* (1
1 mol), A*/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) and AF*/P* (1
1 mol) and AF*/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) at the B97D/6-31G(d,p) level which is shown in Fig. S4† to get a better understanding of its chemical reactivity (the other asymmetric H-bonded dimeric complexes are also shown in Fig. S3 of the ESI†). The charge distributions in molecules can be displayed by electron-rich (red; δ−) and electron-poor (blue; δ+).
1 mol) at the B97D/6-31G(d,p) level which is shown in Fig. S4† to get a better understanding of its chemical reactivity (the other asymmetric H-bonded dimeric complexes are also shown in Fig. S3 of the ESI†). The charge distributions in molecules can be displayed by electron-rich (red; δ−) and electron-poor (blue; δ+).
Conclusions
In this work, a series of novel asymmetric H-bonded dimeric complexes D/P and D/P* (where D = A, A*, AF and AF*) were synthesized and self-assembled by different molar ratios of H-donors (A, A*, AF and AF*) and H-acceptors (P and P*), where the chiral centers were introduced into the linker of H-acceptors (P and P*) and/or the flexible terminus of H-donors (A* and AF*) to study the H-bonded effects on the presence of BPs. In addition, the influences of the lateral fluoro-substituent of H-donors (AF and AF*) on the mesophasic behaviours (e.g., BPs) of asymmetric H-bonded dimeric complexes are investigated. Moreover, H-bonded dimeric complex AF*/P* (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol) with the widest BP range of 6.0 °C in this report can be further optimized to exhibit even wider temperature range of BPI (∼13.2 °C) by a molar ratio of 3
1 mol) with the widest BP range of 6.0 °C in this report can be further optimized to exhibit even wider temperature range of BPI (∼13.2 °C) by a molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 due to the excessive acid dimmer AF* acted as a chiral dopant. Comparing the BPs of H-bonded dimeric complexes A*/P, A/P* and A*/P* with those of our previously reported analogous covalent diads A*-P, A-P* and A*-P*, respectively, the bent angle plays an important role to control the blue phase, which is preferred to exist at the bent angle between 132.1–152.9°. Therefore, due to unsuitable bent angles, both H-bonded dimeric complex A/P* and covalent diad A*-P (with bent angles of 162.0° and 126.5°, respectively) did not possess any blue phase. Finally, the H-bonded effects of chiral centers and lateral fluoro-substituent on supramolecular mesohasic structures via theoretical calculation of biaxiality, bent angle and dipole moment are useful to extend and stabilize the temperature ranges of BPs.
1 due to the excessive acid dimmer AF* acted as a chiral dopant. Comparing the BPs of H-bonded dimeric complexes A*/P, A/P* and A*/P* with those of our previously reported analogous covalent diads A*-P, A-P* and A*-P*, respectively, the bent angle plays an important role to control the blue phase, which is preferred to exist at the bent angle between 132.1–152.9°. Therefore, due to unsuitable bent angles, both H-bonded dimeric complex A/P* and covalent diad A*-P (with bent angles of 162.0° and 126.5°, respectively) did not possess any blue phase. Finally, the H-bonded effects of chiral centers and lateral fluoro-substituent on supramolecular mesohasic structures via theoretical calculation of biaxiality, bent angle and dipole moment are useful to extend and stabilize the temperature ranges of BPs.
Acknowledgements
The financial supports of this project are provided by the Ministry of Science and Technology (MOST) in Taiwan through MOST 103-2113-M-009-018-MY3 and MOST 103-2221-E-009-215-MY3. The powder XRD measurements were supplied by beam-line BL17A (charged by Dr Jey-Jau Lee) of the National Synchrotron Radiation Research Center (NSRRC) in Taiwan.Notes and references
- H. Kikuchi, Struct. Bonding, 2008, 128, 99–117 CrossRef CAS.
- L. Rao, Z. Ge, S. Gauza, K. M. Chen and S. T. Wu, Mol. Cryst. Liq. Cryst., 2010, 527, 30–42 CAS.
- Y. H. Lin, H. S. Chen, H. C. Lin, Y. S. Tsou, H. K. Hsu and W. Y. Li, Appl. Phys. Lett., 2010, 96, 113505 CrossRef.
- Y. H. Lin, H. S. Chen, T. H. Chiang, C. H. Wu and H. K. Hsu, Opt. Express, 2011, 19, 2556–2561 CrossRef CAS PubMed.
- W. Cao, A. Munoz, P. Palffy-Muhoray and B. Taheri, Nat. Mater., 2002, 1, 111–113 CrossRef CAS PubMed.
- J. Yan, L. Rao, M. Jiao, H. C. Cheng and S. T. Wu, J. Mater. Chem., 2011, 21, 7870–7877 RSC.
- S. Yokoyama, S. Mashiko, H. Kikuchi, K. Uchida and T. Nagamura, Adv. Mater., 2006, 18, 48–51 CrossRef CAS.
- F. Castles, F. V. Day, S. M. Morris, D. H. Ko, D. J. Gardiner, M. M. Qasim, S. Nosheen, P. J. W. Hands, S. S. Choi, R. H. Friend and H. J. Coles, Nat. Mater., 2012, 11, 599–603 CrossRef CAS PubMed.
- H.-S. Kitzerow, ChemPhysChem, 2006, 7, 63–66 CrossRef CAS PubMed.
- A. Yoshizawa, RSC Adv., 2013, 3, 25475–25497 RSC.
- O. Jin, D. Fu, J. Wei, H. Yang and J. Guo, RSC Adv., 2014, 4, 28597–28600 RSC.
- H. Stegemeyer, T. Blumel, K. Hiltrop, H. Onusseit and F. Porsch, Liq. Cryst., 1986, 1, 3–28 CrossRef CAS.
- E. Dubois-Violette and B. Pansu, Mol. Cryst. Liq. Cryst., 1988, 165, 151–182 CrossRef CAS.
- J. A. N. Zasadzinski, S. Meiboom, M. J. Sammon and D. W. Berreman, Phys. Rev. Lett., 1986, 57, 364–367 CrossRef CAS PubMed.
- O. Henrich, K. Stratford, M. E. Cates and D. Marenduzzo, Phys. Rev. Lett., 2011, 106, 107801 CrossRef CAS PubMed.
- I. Dierking, W. Blenkhorn, E. Credland, W. Drake, R. Kociuruba, B. Kayser and T. Michael, Soft Matter, 2012, 8, 4355–4362 RSC.
- H. Kikuchi, M. Yokota, Y. Hisakado, H. Yang and T. Kajikawa, Nat. Mater., 2002, 1, 64–68 CrossRef CAS PubMed.
- H. J. Coles and M. N. Pivnenko, Nature, 2005, 436, 997–1000 CrossRef CAS PubMed.
- A. Yoshizawa, Y. Kogawa, K. Kobayashi, Y. Takanishi and J. Yamamoto, J. Mater. Chem., 2009, 19, 5759–5764 RSC.
- M. Sato and A. Yoshizawa, Adv. Mater., 2007, 19, 4145–4148 CrossRef CAS.
- M. Tanaka and A. Yoshizawa, J. Mater. Chem. C, 2013, 1, 315–320 RSC.
- L. Wang, W. He, X. Xiao, F. Meng, Y. Zhang, P. Yang, L. Wang, J. Xiao, H. Yang and Y. Lu, Small, 2012, 8, 2189–2193 CrossRef CAS PubMed.
- S. Taushanoff, K. Van Le, J. Williams, R. J. Twieg, B. K. Sadashiva, H. Takezoe and A. Jákli, J. Mater. Chem., 2010, 20, 5893–5898 RSC.
- I. H. Chiang, C. J. Long, H. C. Lin, W. T. Chuang, J. J. Lee and H. C. Lin, ACS Appl. Mater. Interfaces, 2014, 6, 228–235 CAS.
- H. Chen, Y. Liu, T. Gong, L. Wang, K. Zhao and S. Zhou, RSC Adv., 2013, 3, 7048–7056 RSC.
- Y. Arakawa, S. Kang, J. Watanabe and G. I. Konishi, RSC Adv., 2015, 5, 8056–8062 RSC.
- A. Paikar, A. Pramanik and D. Haldar, RSC Adv., 2015, 5, 31845–31851 RSC.
- W. L. He, M. J. Wei, H. Yang, Z. Yang, H. Cao and D. Wang, Phys. Chem. Chem. Phys., 2014, 16, 5622–5626 RSC.
- Y. Shi, X. Wang, J. Wei, H. Yang and J. Guo, Soft Matter, 2013, 9, 10186–10195 RSC.
- W. He, G. Pan, Z. Yang, D. Zhao, G. Niu, W. Huang, X. Yuan, J. Guo, H. Cao and H. Yang, Adv. Mater., 2009, 21, 2050–2053 CrossRef CAS.
- J. Guo, Y. Shi, X. Han, O. Jin, J. Wei and H. Yang, J. Mater. Chem. C, 2013, 1, 947–957 RSC.
- Y. Li, Y. Cong, H. Chu and B. Zhang, J. Mater. Chem. C, 2014, 2, 1783–1790 RSC.
- C. L. Wei, T. C. Chen, P. Raghunath, M. C. Lin and H. C. Lin, RSC Adv., 2015, 5, 4615–4622 RSC.
- M. J. Frisch, G. W. Trucks and H. B. Schlegel, et al., Gaussian 09, Revision A.1, Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
- S. Grimme, J. Comput. Chem., 2006, 27, 1787–1799 CrossRef CAS PubMed.
- B. H. Besler, K. M. Merz Jr and P. A. Kollman, J. Comput. Chem., 1990, 11, 431–439 CrossRef CAS.
- C. Osuji, C. Y. Chao, I. Bita, C. K. Ober and E. L. Thomas, Adv. Funct. Mater., 2002, 12, 753–758 CrossRef CAS.
- Q. Wei, L. Shi, H. Cao and H. Yang, Liq. Cryst., 2007, 34, 855–860 CrossRef CAS.
- C. W. Wu and H. C. Lin, Macromolecules, 2006, 39, 7985–7997 CrossRef CAS.
- L. Y. Wang, S. Y. Tsa and H. C. Lin, Macromolecules, 2010, 43, 1277–1288 CrossRef CAS.
- P. J. Yang, L. Y. Wang, C. Y. Tang and H. C. Lin, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 764–774 CrossRef CAS.
- T. Kato and J. M. J. Frechet, J. Am. Chem. Soc., 1989, 111, 8533–8534 CrossRef CAS.
- Y. Q. Tian, Y. J. Zhang, Y. Y. Zhao and X. Y. Tang, Chem. J. Chin. Univ., 1997, 18, 1715–1718 CAS.
- T. Kato, J. M. J. Frechet, P. G. Wilson, T. Saito, T. Uryu, A. Fujishima, C. Jin and F. Kaneuchi, Chem. Mater., 1993, 5, 1094–1100 CrossRef CAS.
- M. Hird, Chem. Soc. Rev., 2007, 36, 2070–2095 RSC.
- M. Lee, S. T. Hur, H. Higuchi, K. Song, S. W. Choi and H. Kikuchi, J. Mater. Chem., 2010, 20, 5813–5816 RSC.
- I. Dierking and P. Archer, RSC Adv., 2013, 3, 26433–26437 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra06868a |
| This journal is © The Royal Society of Chemistry 2015 |