A novel lithium–sulfur battery cathode from butadiene rubber-caged sulfur-rich polymeric composites†
Abstract
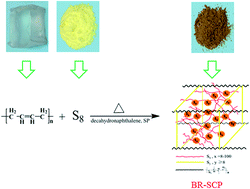
Novel sulfur-rich polymeric materials were readily prepared via facile solution vulcanization of the commercial butadiene rubber (BR) and sulfur element, and were investigated as cathode materials for lithium–sulfur batteries. During the solution vulcanization procedure, the double bonds (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) in butadiene rubber are chemically cross-linked with sulfur. Moreover, the sulfur canalso self-polymerize into polymeric sulfur with long molecular chain. The polymeric sulfur chains penetrate into the cross-linked BR network to form a unique semi-internal penetration network (semi-IPN) confinement structure, which can effectively alleviate the dissolution and diffusion of intermediate polysulfide into electrolytes. Meanwhile, the obtained sulfur-rich polymeric composites have high sulfur contents even over 90%. As a result, the as-prepared sulfur-rich polymeric composites (BR-SPC) with network confine caged structure exhibit excellent cycling stability and high coulombic efficiency. An initial discharge specific capacity of 811 mA h g−1 is reached, and retains 671 mA h g−1 after 50 cycles at 0.1 C. The capacity retention rate and coulombic efficiency are 83%, 100%, respectively. Additionally, Super P (carbon black) was added in situ before vulcanization to increase the conductivity of BR-SPC composites. The BR-SPC composite containing Super P (BR-SPC-SP) reveals higher capacity retention of 85% over 50 cycles at 0.5 C than the BR-SPC composite without Super P.
C) in butadiene rubber are chemically cross-linked with sulfur. Moreover, the sulfur canalso self-polymerize into polymeric sulfur with long molecular chain. The polymeric sulfur chains penetrate into the cross-linked BR network to form a unique semi-internal penetration network (semi-IPN) confinement structure, which can effectively alleviate the dissolution and diffusion of intermediate polysulfide into electrolytes. Meanwhile, the obtained sulfur-rich polymeric composites have high sulfur contents even over 90%. As a result, the as-prepared sulfur-rich polymeric composites (BR-SPC) with network confine caged structure exhibit excellent cycling stability and high coulombic efficiency. An initial discharge specific capacity of 811 mA h g−1 is reached, and retains 671 mA h g−1 after 50 cycles at 0.1 C. The capacity retention rate and coulombic efficiency are 83%, 100%, respectively. Additionally, Super P (carbon black) was added in situ before vulcanization to increase the conductivity of BR-SPC composites. The BR-SPC composite containing Super P (BR-SPC-SP) reveals higher capacity retention of 85% over 50 cycles at 0.5 C than the BR-SPC composite without Super P.

 Please wait while we load your content...
Please wait while we load your content...