Preparation and catalytic applications of nanomaterials: a review
Abstract
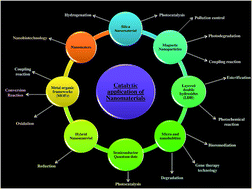
Catalysts play a very important role in the chemical industries. Catalysts have been used in processes like the workup of fuels such as oil, gas and coal, purification of effluents and industrial waste gases etc. Heterogeneous catalysts are gaining much attention compared to homogeneous catalysts as they confer more selectivity and provide better yield. Research in new catalytic materials or optimization of existing catalyst systems is of enormous importance in order to increase the efficiency of the catalyst, resulting in higher product yields and purities. Currently, the research is more focused towards nanostructured catalysts with enhanced physiochemical properties. Nanoscale catalysts have high specific surface area and surface energy, which ultimately lead to the high catalytic activity. Nano-catalysts improve the selectivity of the reactions by allowing reaction at a lower temperature, reducing the occurrence of side reactions, higher recycling rates and recovery of energy consumption. Therefore, these are widely used in green chemistry, environmental remediation, efficient conversion of biomass, renewable energy development and other areas of interest. In this review the prospects, paradox and perspective of the preparation and catalytic application of nanomaterials in organic synthetic chemistry is reviewed, and an outlook of their developments is discussed.

 Please wait while we load your content...
Please wait while we load your content...