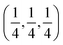DOI:
10.1039/C5RA06730H
(Paper)
RSC Adv., 2015,
5, 50186-50195
Vacancy mediated ionic conduction in Dy substituted nanoceria: a structure–property correlation study
Received
14th April 2015
, Accepted 21st May 2015
First published on 22nd May 2015
Abstract
The oxygen vacancy evolution and ion dynamics in Dy doped nanoceria have been investigated with microstructural, optical and ionic conductivity studies. The influence of Dy3+ ions on the microstructures and the optical and ionic conductivity properties of these nanoparticles has been studied using X-ray diffraction, HR-TEM, EDAX, UV-vis, Raman and impedance spectroscopy. From Rietveld refinement of the XRD profiles, it has been found that the oxygen vacancies, lattice parameters and Ce–O bond lengths increase with Dy3+ ion concentration. The Rietveld analysis, together with HR-TEM, confirms the cubic fluorite structure with space group Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m of all the samples. The EDAX spectra show good stoichiometry of the different atoms in the samples. The direct band gap, calculated from UV-vis spectra, shows a red shift with increasing concentration of Dy3+ ions. Raman spectroscopy studies of the samples gave insight into their vibrational properties and pointed to the fact that the oxygen vacancy content increased significantly with the doping concentration. The number of oxygen vacancies and their interaction with dopant cations strongly influence the electrical properties of Dy doped ceria.
m of all the samples. The EDAX spectra show good stoichiometry of the different atoms in the samples. The direct band gap, calculated from UV-vis spectra, shows a red shift with increasing concentration of Dy3+ ions. Raman spectroscopy studies of the samples gave insight into their vibrational properties and pointed to the fact that the oxygen vacancy content increased significantly with the doping concentration. The number of oxygen vacancies and their interaction with dopant cations strongly influence the electrical properties of Dy doped ceria.
1. Introduction
The ionic conductivity of ceria based solid solutions has been reported to be an order of magnitude greater than that of yttrium-stabilized zirconia (YSZ) when it is in nanocrystalline form.1 Ceria based nanomaterials have also found applications as catalytic supports in automotive exhaust systems.2 Rare earth elements are praised as valuable materials due to their special optical, electrical and magnetic properties, which are attributed to their particular electron configurations.3 When ceria is doped with rare earth elements, the substitution of Ce4+ in ceria by these trivalent cations distorts the lattice structure and generates oxygen vacancies,4 which permits high oxygen ion conduction. The oxygen vacancy concentration of CeO2 can be varied by varying the dopant concentration.5 Rare earth doped ceria is also a good candidate as a wavelength converter of near UV photons to IR photons.6 A comprehensive knowledge of the crystal structures of these compounds and a better understanding of their mechanism of ionic conduction are therefore required to develop better electrolyte materials. Hence, in order to use rare earth doped ceria as electrolyte materials, many researchers have studied the crystal structures, ordering of oxygen vacancies and diffusion paths of these ionic conductors.7–10 The movements of these oxygen vacancies are influenced by their interactions with dopant cations. A relatively small concentration of point defects may affect the physical properties of these materials in very significant ways.11 Raman spectroscopy is an essential tool to investigate this type of local structural distortion.12 The main difficulty for the application of rare earth doped ceria in SOFC electrolytes is the creation of both electronic and ionic charge carriers at low oxygen partial pressures. The ionic conduction in rare earth doped ceria is dominated by the association between the trivalent cations and the oxygen vacancies. Among the various rare earth doped ceria materials, ceria doped with Sm3+ and Gd3+ shows higher ionic conductivity because of the optimal radii of these elements and, therefore, their smaller association enthalpy.13,14 Recent studies15 have also pointed out that Dy doped ceria may be a promising solid electrolyte material for applications in low temperature SOFCs.
In our present work, Dy doped Ce1−xDyxO2−δ (0.0 ≤ x ≤ 0.5) nanoparticles were prepared using the citrate auto-ignition method. The detailed microstructures and the optical properties of these nanoparticles were investigated. The effect of dysprosium doping on the evolution of oxygen vacancies with respect to microstructure and optical properties, and the dependence of electrical conductivity on the doping concentration and oxygen vacancies, are discussed and correlated.
2. Materials and methods
The Dy doped ceria nanomaterials Ce1−xDyxO2−δ (0.0 ≤ x ≤ 0.5) were prepared by using a low temperature citrate auto-ignition method. Ce(NO3)3·6H2O (99.9%) and Dy2O3 (99.9%) were used as starting materials. The process of sample preparation has been described in our previous work.16 The as prepared powdered samples were annealed at 400 °C for 2 h, then sintered at 600 °C for 6 h. During annealing/sintering the temperature was increased at the rate of 5 °C min−1, and the materials were then cooled to room temperature by normal furnace cooling. To identify the crystal structures and phase purity of the samples, X-ray diffraction profiles were recorded with a powder X-ray diffractometer (BRUKER, Model D8 Advance-AXS) using CuKα radiation [ λ = 1.5406 Å] from 2θ = 20° to 90° with a step size of 0.05°. The microstructural analyses at high magnification were performed by placing the particles in a Formvar-carbon coated 300 mesh copper grid with the help of a transmission electron microscope (JEOL, Model JEM-2010) operated at 200 kV. The TEM micrographs were further analyzed using the Gatan microscopy suite. The compositions of the samples were evaluated by energy dispersive X-ray analysis (Hitachi S-3500). Ultraviolet-visible (UV-vis) absorption spectra were taken at room temperature in the wavelength range of 200–1100 nm using a Shimadzu spectrophotometer (Model-1800). Raman spectra of the materials were recorded at room temperature using a Triple Raman Spectrometer (Jobin-Yvon Horiba T64000) equipped with a TE cooled charge coupled-device detector and an Olympus microscope using a He–Ne laser at the 632.817 nm line as the excitation source in the wavenumber range of 200–800 cm−1. For the electrical measurements, cylindrical pellets were prepared from sintered powder by uniaxial pressing in a 10 mm diameter stainless steel die. The pellets were covered on both sides with conductive graphite paste to make the electrodes. The electrical measurements were performed using two probe methods in air. A LCR meter (HIOKI, Model 3532-50) interfaced with a PC was used to collect the electrical data in the frequency range of 42 Hz–5 MHz and in the temperature range of 250–550 °C.
3. Results and discussion
3.1. X-Ray diffraction analysis and Rietveld refinement
Fig. 1(a) shows the XRD pattern of the Ce1−xDyxO2−δ (x = 0.00–0.50) samples. It may be found that when ceria is doped with Dy, there is no noticeable change in the diffraction pattern, i.e. any additional peak due to Dy is absent. This confirms the complete dissolution of Dy into the ceria lattice. The observed peaks in the XRD pattern were well indexed and consistent with the reference data [JCPDS file: 34-03940]. The observed Bragg reflection peaks confirmed the cubic fluorite structure of the samples. We have plotted the maximum intensity peak (111) against the concentration of Dy in Fig. 1(b). It can be seen from Fig. 1(b) that the peak shifts towards the lower angle side with increasing doping concentration (x). This shift of the (111) peak clearly indicates the lattice expansion with Dy content in the ceria lattice.
 |
| | Fig. 1 (a) XRD patterns of the doped and undoped ceria Ce1−xDyxO2−δ (x = 0.00–0.50). (b) Shift of (111) peak shift with doping concentration x. The refined XRD patterns obtained from Rietveld analysis of the samples for (c) x = 0.20 and (d) x = 0.50. | |
To obtain microstructural information for the samples, we have performed Rietveld analysis of the XRD data using MAUD 2.33 software, which is specially designed to simultaneously refine both the structural and microstructural parameters through a least squares method. Fig. 1(c) and (d) show the refined XRD pattern obtained from Rietveld analysis for the samples x = 0.20 and x = 0.50 respectively. The shape of the diffraction profile is fitted with a pseudo-Voigt function (described by Gaussian + Lorentzian functions with a refinable degree of mixing) with asymmetry because this function takes individual results into account for both the particle size and strain broadening of the experimental data.17 The angular dependence of the peak full width at half maximum (FWHM) is described by Caglioti's formula. The background of each XRD profile is fitted using a polynomial of degree 5. The difference between the observed and simulated diffraction patterns was minimized using the Marguardt least-squares procedure. This minimization was carried out using reliability index parameters such as weighted residual error (Rwp), expected error (Rexp) and goodness of fit (GoF).18–20 These parameters are defined as:
| |
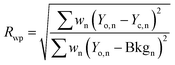 | (1) |
| |
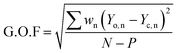 | (2) |
where
Yo,n and
Yc,n are the observed and calculated data at point n, respectively; Bkg
n is the background at data point n;
N is the number of data points;
P is the number of parameters; and
wn is the weighting factor given to data point n. In counting statistics, this last factor is given by
wn = 1/
σ(
Yo,n)
2, where
σ(
Yo,n) is the error in
Yo,n. Both
Rwp and GoF are good global indicators of the refinement process, since the numerators of these factors contain the residual function which is being minimized. A desirably good refinement is represented by the low values of these parameters:
Rwp around 0.10 for XRD in a conventional diffractometer, and GoF around 1.
21 In all refinements, GoF is between 1.1 and 1.2, which indicates the success of the refinement. Different Reitveld parameters, such as
Rexp,
Rwp and GoF, for each XRD profile are listed in
Table 1. We have evaluated the effective particle size (
D) and RMS strain (〈
ε2〉
1/2) using the Popa model.
22 Other structural parameters such as lattice parameter, Ce–O bond length, atomic position and occupancies were calculated and are given in
Table 1.
Table 1 Rietveld refinement analysis results and theoretical densities for the Ce1−xDyxO2−δ samples (x = 0.00–0.50). The errors after the fourth decimal are indicated inside parentheses
| Ce1−xDyxO2−δ (x = 0–0.5) |
x = 0.00 |
x = 0.10 |
x = 0.15 |
x = 0.20 |
x = 0.25 |
x = 0.30 |
x = 0.40 |
x = 0.50 |
| Crystal system |
Cubic |
Cubic |
Cubic |
Cubic |
Cubic |
Cubic |
Cubic |
Cubic |
| Space group |
Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
| Atomic co-ordinate |
| Ce(4a) |
| (x,y,z) |
(0,0,0) |
(0,0,0) |
(0,0,0) |
(0,0,0) |
(0,0,0) |
(0,0,0) |
(0,0,0) |
(0,0,0) |
| Occupancy |
0.9989(7) |
0.9106(1) |
0.8499(5) |
0.8039(3) |
0.7632(2) |
0.7155(1) |
0.6265(7) |
0.5091(3) |
| Dy(4a) |
| (x,y,z) |
— |
(0,0,0) |
(0,0,0) |
(0,0,0) |
(0,0,0) |
(0,0,0) |
(0,0,0) |
(0,0,0) |
| Occupancy |
— |
0.0894(5) |
0.1501(3) |
0.1961(8) |
0.2368(4) |
0.2845(4) |
0.3735(7) |
0.4909(2) |
| O(8c) |
| (x,y,z) |
 |
 |
 |
 |
 |
 |
 |
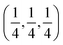 |
| Occupancy |
0.9859(1) |
0.9482(5) |
0.9334(8) |
0.8898(6) |
0.8813(2) |
0.8634(1) |
0.8176(1) |
0.7698(5) |
| Lattice parameter (Å) |
5.3976(9) |
5.4019(1) |
5.4045(5) |
5.4064(4) |
4.4085(3) |
5.4101(1) |
5.4117(8) |
5.4138(0) |
| Particle size (nm) |
14.86 |
21.65 |
16.67 |
13.88 |
13.49 |
13.78 |
20.16 |
21.53 |
| RMS strain |
1.25 × 10−5 |
8.33 × 10−4 |
2.25 × 10−4 |
1.25 × 10−5 |
7.68 × 10−5 |
3.14 × 10−4 |
1.25 × 10−4 |
1.07 × 10−3 |
| Ce–O bond length (Å) |
2.3372(7) |
2.3391(0) |
2.3402(4) |
2.3410(6) |
2.3419(6) |
2.3426(5) |
2.3433(7) |
2.3442(4) |
| Density (g cm−3) |
7.268 |
7.312 |
7.332 |
7.354 |
7.376 |
7.399 |
7.453 |
7.505 |
| Rwp (%) |
5.42 |
4.34 |
5.26 |
5.79 |
3.69 |
3.61 |
4.34 |
5.03 |
| Rexp (%) |
4.63 |
3.91 |
4.69 |
5.03 |
3.29 |
3.11 |
3.67 |
4.37 |
| GOF |
1.17 |
1.11 |
1.12 |
1.15 |
1.12 |
1.16 |
1.18 |
1.15 |
The Rietveld analysis indicated that each sample had the cubic fluorite structure with space group Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. In this structure, cerium ions occupy the vertices and face of the cubic unit cell. Each cerium ion (Ce4+) is co-ordinated with eight oxygen ions (O2−) arranged in a perfect cube, while each oxygen ion is surrounded by four cerium ions in a tetrahedral arrangement. This structure is often described as a cubic closed packing of cerium ions with oxygen ions occupying all tetrahedral holes.23 The crystal structure of pure ceria is shown in Fig. 2(a). All the cerium ions (Ce4+) are situated at site 4a with the atomic co-ordinate (0,0,0), and the oxygen ions (O2−) are at site 8c, corresponding to the (1/4, 1/4, 1/4) position. All the ion positions are fixed during the whole refinement process. Generally, when the fluorite structured ceria is doped with a trivalent cation such as Dy3+, one oxygen vacancy is formed for every two trivalent cations for charge neutrality, which is represented by Kröger–Vink notation:
m. In this structure, cerium ions occupy the vertices and face of the cubic unit cell. Each cerium ion (Ce4+) is co-ordinated with eight oxygen ions (O2−) arranged in a perfect cube, while each oxygen ion is surrounded by four cerium ions in a tetrahedral arrangement. This structure is often described as a cubic closed packing of cerium ions with oxygen ions occupying all tetrahedral holes.23 The crystal structure of pure ceria is shown in Fig. 2(a). All the cerium ions (Ce4+) are situated at site 4a with the atomic co-ordinate (0,0,0), and the oxygen ions (O2−) are at site 8c, corresponding to the (1/4, 1/4, 1/4) position. All the ion positions are fixed during the whole refinement process. Generally, when the fluorite structured ceria is doped with a trivalent cation such as Dy3+, one oxygen vacancy is formed for every two trivalent cations for charge neutrality, which is represented by Kröger–Vink notation:
| |
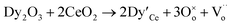 | (3) |
 |
| | Fig. 2 The crystal structure of (a) pure ceria and (b) doped ceria. | |
Here Dy′Ce indicates one Ce4+ site occupied by a Dy3+ ion, and  represents the oxygen vacancy. The defect structure of the doped ceria is shown in Fig. 2(b). Here, the occupancy of Ce4+ ions in the 4a site and O2− ions in the 8c site of undoped ceria are 0.9989(7) and 0.9859(1) respectively (Table 1). In doped ceria, the occupancy of Ce4+ ions decreases with the doping concentration, and the occupancy of dopant ions (Dy3+) in the 4a site increases. The occupancy of O−2 ions at the 8c site decreases with the doping concentration of Dy3+ ions, which indicates a higher number of oxygen vacancies. The formation of these oxygen vacancies has been confirmed in earlier studies.24–27 Fig. 3 shows the variation of the lattice parameters of the Ce1−xDyxO2−δ (x = 0.00–0.50) samples as a function of total dopant concentration (x). It can be seen that the lattice parameter increases with dopant concentration, which is due to the fact that the ionic radius of Dy3+ (1.027 Å) is greater than the ionic radius of Ce4+ (0.97 Å). It can be seen from Fig. 3 that for the Ce1−xDyxO2−δ system, the lattice parameter increases linearly with increasing Dy3+ concentration up to x = 0.25, following Vegard's law.28 After x = 0.25, the lattice parameter of the Ce1−xDyxO2−δ system also increases linearly, but the rate of increase is different. Using a least-squares fitting algorithm, a linear relationship was obtained between the lattice parameters (a) and dopant concentration (x). These can be represented as
represents the oxygen vacancy. The defect structure of the doped ceria is shown in Fig. 2(b). Here, the occupancy of Ce4+ ions in the 4a site and O2− ions in the 8c site of undoped ceria are 0.9989(7) and 0.9859(1) respectively (Table 1). In doped ceria, the occupancy of Ce4+ ions decreases with the doping concentration, and the occupancy of dopant ions (Dy3+) in the 4a site increases. The occupancy of O−2 ions at the 8c site decreases with the doping concentration of Dy3+ ions, which indicates a higher number of oxygen vacancies. The formation of these oxygen vacancies has been confirmed in earlier studies.24–27 Fig. 3 shows the variation of the lattice parameters of the Ce1−xDyxO2−δ (x = 0.00–0.50) samples as a function of total dopant concentration (x). It can be seen that the lattice parameter increases with dopant concentration, which is due to the fact that the ionic radius of Dy3+ (1.027 Å) is greater than the ionic radius of Ce4+ (0.97 Å). It can be seen from Fig. 3 that for the Ce1−xDyxO2−δ system, the lattice parameter increases linearly with increasing Dy3+ concentration up to x = 0.25, following Vegard's law.28 After x = 0.25, the lattice parameter of the Ce1−xDyxO2−δ system also increases linearly, but the rate of increase is different. Using a least-squares fitting algorithm, a linear relationship was obtained between the lattice parameters (a) and dopant concentration (x). These can be represented as
| | |
a(x, 0 ≤ x ≤ 0.25) = 5.39771 + 0.04369x
| (4) |
and
| | |
a(x, 0.3 ≤ x ≤ 0.5) = 5.40451 + 0.01847x
| (5) |
 |
| | Fig. 3 Variation of the lattice parameter with doping concentration x. | |
Similar results were found earlier for the Ce1−xGdxO2−δ (0.05 ≤ x ≤ 0.4) system.29 In the present study, the slower increase of the lattice parameters at higher concentrations (x > 0.25) can be attributed to the effect of the interaction between the dopant cations and the oxygen vacancies, which tends to contract the unit cell.30 As the oxygen vacancy increases with doping concentration x, at higher doping concentrations the number of oxygen vacancies is much greater. Therefore, the interaction between the oxygen vacancies and dopant ions is greater at higher concentrations. At higher doping concentrations, the oxygen vacancies and defect associations appearing in the solid solutions are expected to have different interactions with the network ions, and the oxygen vacancy is believed to produce a lattice contraction rather than a defect association.31 The RMS strain for the sample Ce0.8Dy0.2O2−δ shows a minimum value of 1.25 × 10−5. The Ce–O bond length of the system was also evaluated by Rietveld analysis and was found to increase with x, i.e. the change in the lattice parameter demonstrates the same behavior as the Ce–O bond length change. During the formation of rare earth doped ceria solid solutions, several defect reactions can occur. Among these, the oxygen vacancy model is very important, and is represented by the Kröger–Vink notation as given earlier. With the help of the oxygen vacancy model of doped ceria, we can determine the density of the system Ce1−xDyxO2−δ (x = 0.00–0.50) using the following equation:
| |
 | (6) |
where
MDy,
MCe and
MO are the atomic weights of Dy, Ce and oxygen, repectively;
NA is Avogadro's number, and
a is the lattice parameter. The values of the densities are given in
Table 1 and are found to increase with doping concentration, as the atomic weight of Dy (162.5) is greater than that of Ce (140.11).
3.2. Energy dispersive X-ray analysis
The composition of the obtained system was analyzed by means of energy dispersive X-ray analysis (EDAX). Fig. 4(a)–(d) show the EDAX spectra for the compositions x = 0.00, x = 0.10, x = 0.20 and x = 0.50 respectively. The presence of major chemical elements, namely cerium, dysprosium and oxygen, in the prepared samples was confirmed from this analysis. The percentages of the Dy/Ce values are given in the inset of Fig. 4(a)–(d). The doped ceria did not deviate from their initial stoichiometry and matched well with the initial degree of Dy substitution. The EDAX spectrum clearly shows that oxygen content in the Ce1−xDyxO2−δ system decreases with doping concentration, i.e. the oxygen vacancies increase with doping concentration. This result is in good agreement with the Rietveld analysis results discussed earlier.
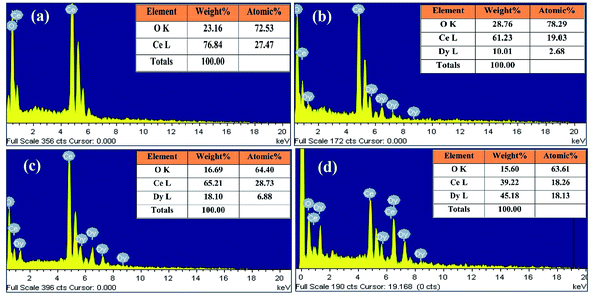 |
| | Fig. 4 Energy dispersive X-ray analysis (EDAX) for (a) x = 0.00, (b) x = 0.10, (c) x = 0.20 and (d) x = 0.50. The percentage of Dy/Ce values are given in the inset. | |
3.3. Transmission Electron Microscopy (TEM) studies
The nanostructures of the samples were further characterized by transmission electron microscopy. The nanoflakes for the sample x = 0.2 are distributed uniformly, as observed in the bright field TEM image of Fig. 5(a). All the samples show similar nanostructures, with negligible size variation with composition. The crystallite size distribution for the sample x = 0.2 is shown in the inset of Fig. 5(a). It can be inferred that the average crystallite size is ∼16.84 nm, indicating that the sample preparation is successful in producing such nanostructures. In Fig. 5(b), the lattice fringe pattern is shown for the composition x = 0.2. The fast Fourier transform (FFT) of the embraced zone is shown in the inset of Fig. 5(b). At least three different pairs of bright spots are found, and these spots are identified as reflections from the (111), (002) and (11−1) lattice planes, as shown in the inset of Fig. 5(b). The sharp and bright spots further confirmed the cubic symmetry and good crystallinity of the sample. Using SAED studies, D. R. Ou et al. have shown an increase in the local ordering of oxygen vacancies with increasing doping concentration in lanthanide doped ceria.32 In Fig. 5(c) the simulated lattice pattern is also shown, with prominent lattice planes orientated along the (111) direction. It can be inferred that the lattice fringe in Fig. 5(c) consists of the (111), (002) and (11−1) planes, as previously suggested by the FFT pattern. To obtain more information, we have analyzed the atomic model using the XRD refinement results. Fig. 5(c) contains all of these lattice planes with a viewing direction along [−110], as suggested by the atomic model in Fig. 5(d). It can be noted that Dy3+ is partially occupying the Ce4+ site, so these cations cannot be isolated; however, the position of the cation (Ce4+/Dy3+) is detectable, as observed in Fig. 5(c) and (d).
 |
| | Fig. 5 (a) Indicates the distribution of nanoflakes for the composition x = 0.2. The crystallite size distribution is also shown in the inset of (a). In (b) the lattice plane is shown. The FFT image is also shown in the inset of (b). The simulated lattice pattern is shown in (c). In (d) the atomic model indicates the orientation of the atoms. The gray spheres in (d) indicate the Ce4+/Dy3+ atoms, whereas darker spheres are O2−. | |
3.4. UV-vis spectroscopy
Fig. 6(a) shows the UV-VIS absorption spectra recorded for pure and doped ceria. It can be seen that there is a strong absorption band below 400 nm in the spectra for all the samples, which is due to the charge transfer from O−2(2p) to Ce4+(4f) orbitals in CeO2.33 However, there is no absorption in the visible region. The optical band gap values for the Ce1−xDyxO2−δ (x = 0.00–0.50) system can be estimated from the absorption spectra following Tauc's rule:34where α is the absorption co-efficient, hν is the photon energy, Eg is the band gap energy, A is a constant and n can have values of 1/3, 1/2, 2, and 3 for the direct forbidden, direct allowed, indirect allowed, and indirect forbidden transitions respectively. The band gap corresponding to the direct transition was obtained by extrapolating the linear portions of (αhν)2 versus the hν curves to (αhν)2 equal to zero. A plot of (αhν)2 as a function of photon energy hν for the sample Ce0.75Dy0.25O2−δ is shown in Fig. 6(b). The calculated values of Eg for all the samples are given in Table 2. Undoped ceria shows the highest band gap value of 2.750 eV, and for Dy3+ doped ceria the values of the band gap decrease with dopant concentration x. Thus, the absorption spectra of Dy3+ doped ceria nanocrystals exhibit red shifts compared to that of pure ceria. In CeO2, all valance Ce states, including the 4f states, are empty, and the system is a wide gap insulator with a measured fundamental band gap of 6.0 eV between the valance and conduction bands; this is formed predominantly by the O−2 (2p) to Ce4+ (5d) states respectively.35 The vacant 4f states lie in the gap. However, in our present study, the band gap value of CeO2 is much lower than 6.0 eV. This is because a small amount of Ce3+ is present at the surface of CeO2 36 and one electron per Ce atom populates a Ce 4f state, resulting in a decrease in the band gap value of ceria. The co-existence of a small amount of Ce3+ ions was also confirmed by XPS analysis.37 The red shift of the direct band gap with x can be explained in several ways. Firstly, when Dy3+ ions are incorporated into the ceria lattice, the valency changes from Ce4+ to Ce3+ ions decrease due to the replacement of Ce3+ ions by trivalent Dy3+ ions, as confirmed by several authors using XPS.38–40 The substitution of Ce3+ ion with Dy3+ ion increases with doping concentration, and this substitution increases the oxygen vacancy concentration due to a charge compensation mechanism, as discussed earlier. Secondly, the red shift may be due to the presence of oxygen defect levels between the O2p and Ce4f levels that capture the excited electrons and decrease the effective band gap.41 Lastly, the doping of Dy ions creates ground and excited f-energy states in the mid band gap of ceria. These energy states of Dy accept many of the excited electrons coming from the O2p level.11 This ultimately leads to effective reduction in the band gap, i.e. a red shift.
 |
| | Fig. 6 (a) The UV-VIS absorption spectra for pure and doped ceria Ce1−xDyxO2−δ (x = 0.00–0.50) and (b) the plot of (αhν)2 as a function of photon energy hν for the sample Ce0.8Dy0.2O2−δ. | |
Table 2 The values of the band gap (Eg) for the Ce1−xDyxO2−δ samples (x = 0.00–0.50). The errors after the third decimal are indicated inside parentheses
| Samples |
Band gap (eV) |
| CeO2 |
2.750(5) |
| Ce0.9Dy0.1O2−δ |
2.714(1) |
| Ce0.85Dy0.15O2−δ |
2.685(4) |
| Ce0.8Dy0.2O2−δ |
2.590(3) |
| Ce0.75Dy0.25O2−δ |
2.529(2) |
| Ce0.7Dy0.3O2−δ |
2.516(1) |
| Ce0.6Dy0.4O2−δ |
2.511(8) |
| Ce0.5Dy0.5O2−δ |
2.471(5) |
3.5. Raman spectroscopy
The Raman spectra of sintered samples Ce1−xDyxO2−δ (x = 0.0–0.5) are shown in Fig. 7. These spectra exhibit a very intense Raman band centered at 462 cm−1 to 484 cm−1, as listed in Table 3. These bands were attributed to the Raman-active vibrational mode F2g of the fluorite-type structure, which can be viewed as a symmetrical stretching vibration of oxygen atoms around the Ce3+ ions42 and is sensitive to the crystalline symmetry.43 Table 3 also reveals that the F2g mode shifts towards higher wavenumbers with increasing concentration of Dy3+ cations (i.e. with x). This shift is due to the increase in the number of oxygen vacancies, i.e. the decrease in the amount of oxygen ions bound to Ce or Dy ions.44 Therefore, the number of oxygen vacancies increases in the Ce1−xDyxO2−δ system (x = 0.0–0.5) with x, and this is in good agreement with the Rietveld analysis result. A second order Raman band below 400 cm−1 and above 500 cm−1 was observed, which is due to the extrinsic oxygen vacancies introduced into the ceria when the Ce4+ ions are replaced by Dy3+ ions. These Raman bands are also due to defect spaces such as M4Ov and O6Ov type complexes (M = metal ion and Ov = oxygen vacancy), where an M4Ov type complex consists of an oxygen vacancy surrounded by four nearest neighbor metal ions and an O6Ov type complex consists of an oxygen vacancy surrounded by six next nearest neighbor oxygen ions. These second order Raman bands below 400 cm−1 were also observed in earlier studies.45 The relative intensity of these bands also increases with the doping concentration of Dy3+ cations. For pure ceria, a single intense band centered at 462 cm−1 is observed, which signifies that there is apparently no extrinsic oxygen vacancy. As shown in Fig. 7, for all the doped samples, there is a D band above 500 cm−1 which splits into D1 and D2 bands for the samples x = 0.1–0.4. The D1 and D2 bands were merged into a broad single band for the sample x = 0.5. The center of the D1 and D2 bands shifts towards higher wavenumbers with the concentration of Dy3+ cations. These disordered D1 and D2 bands were used as a powerful tool to investigate the association of the formation of defects with the decrease in the total lattice energy in ceria based materials.46 Hence, the D2 bands from 597 cm−1 to 610 cm −1 are attributed to defect spaces with Oh symmetry, which include a Dy3+ atom in 8-fold co-ordination of O2− but do not contain any O2− vacancies. The D1 bands from 552 cm−1 to 576 cm−1 were assigned to defect spaces with symmetries other than Oh symmetry, including O2− vacancies in the  and
and  complexes. Both the D1 and D2 bands shift towards higher wavenumbers with increasing concentration of Dy3+ ions, and the shift of D1 is assigned to the increase of the
complexes. Both the D1 and D2 bands shift towards higher wavenumbers with increasing concentration of Dy3+ ions, and the shift of D1 is assigned to the increase of the  complex compared to that of the
complex compared to that of the 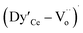 complex. The ratio of the intensities of the different bands is listed in Table 3. The ratio of IF2g/ID1 and IF2g/ID2 decreases with increasing x, which is related to the degree of defect sites on CeO2. This also indicates a higher number of oxygen vacancies with higher doping concentrations.47 According to Nakajima et al.,48 a low ratio of ID1/ID2 indicates that Ce3+ ions preferentially locate in a Ce3+O8 type complex, and a higher value of this ratio indicates an increase in the concentration of the amalgamated defects of O2− vacancies in Dy3+O8 type complexes. S. F. Wang et al.49 and Z. D. Dohčević-Mitrović et al.50 have also shown the increase of oxygen vacancies with doping concentration in rare earth doped ceria solid solutions using Raman spectroscopy. The shift of the Raman bands and the change in the intensity ratio with doping concentration also confirms the formation of solid solutions in our present study by the citrate auto-ignition method.
complex. The ratio of the intensities of the different bands is listed in Table 3. The ratio of IF2g/ID1 and IF2g/ID2 decreases with increasing x, which is related to the degree of defect sites on CeO2. This also indicates a higher number of oxygen vacancies with higher doping concentrations.47 According to Nakajima et al.,48 a low ratio of ID1/ID2 indicates that Ce3+ ions preferentially locate in a Ce3+O8 type complex, and a higher value of this ratio indicates an increase in the concentration of the amalgamated defects of O2− vacancies in Dy3+O8 type complexes. S. F. Wang et al.49 and Z. D. Dohčević-Mitrović et al.50 have also shown the increase of oxygen vacancies with doping concentration in rare earth doped ceria solid solutions using Raman spectroscopy. The shift of the Raman bands and the change in the intensity ratio with doping concentration also confirms the formation of solid solutions in our present study by the citrate auto-ignition method.
 |
| | Fig. 7 Raman spectra of sintered samples Ce1−xDyxO2−δ (x = 0.0–0.5). | |
Table 3 Positions of different Raman bands and intensity ratios of different Raman bands for the Ce1−xDyxO2−δ samples (x = 0.00–0.50)
| Samples |
Positions of Raman bands (cm−1) |
IF2g/ID1 |
IF2g/ID2 |
ID1/ID2 |
| F2g |
D1 |
D2 |
| CeO2 |
462 |
— |
— |
— |
— |
— |
| Ce0.9Dy0.1O2−δ |
463 |
552 |
597 |
12.81 |
14.22 |
1.09 |
| Ce0.8Dy0.2O2−δ |
465 |
558 |
599 |
6.00 |
7.53 |
2.24 |
| Ce0.7Dy0.3O2−δ |
467 |
563 |
600 |
3.13 |
3.76 |
1.20 |
| Ce0.6Dy0.4O2−δ |
472 |
571 |
605 |
2.01 |
2.19 |
1.08 |
| Ce0.5Dy0.5O2−δ |
484 |
576 |
610 |
1.51 |
1.51 |
1.00 |
3.6. Electrical conductivity
Fig. 8(a) shows the variation of the real part σ′(ω) of complex conductivity as a function of frequency at a temperature of 525 °C for the Ce1−xDyxO2−δ system (0.0 ≤ x ≤ 0.5). This figure reveals that conductivity spectra of this system consists of two parts, namely a frequency independent part in the low frequency region which corresponds to the DC conductivity caused by random hopping of the ions, and a frequency dependent part in the high frequency region which corresponds to the AC conductivity caused by the correlated forward backward hopping motion of the charge carriers among the localized sites.51 Therefore, the conductivity spectra can be well described by the following equation:
 |
| | Fig. 8 (a) The variation of the real part σ′(ω) of complex conductivity as a function of frequency at a temperature of 525 °C for all the samples, (b) the variation of σ(0) with doping concentration at different temperatures and (c) Arrhenius plot of the system Ce1−xDyxO2−δ (x = 0.10–0.50). | |
This spectrum also shows the similar nature of all the compositions. The Random free-energy Barrier Model (RBM), proposed by Dyre,52 describes the frequency dependent conductivity over a wide range of frequencies in disordered solids at constant temperature. This model is based on the determination that DC conductivity is more thermally activated than AC conductivity. We used RBM to evaluate the DC conductivity or the total conductivity. According to RBM, the complex conductivity σ*(ω) is given by
| |
 | (9) |
where
σ(0) is the DC conductivity and
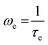
is the attempt frequency to overcome the highest free energy barrier. The real part of
σ*(
ω) has been used to fit the conductivity spectra, as shown in
Fig. 8(a). The variation of
σ(0) with the doping concentration of Dy
3+ for different temperatures is shown in
Fig. 8(b). This figure reveals that the DC conductivity increases with doping concentration and shows a maximum value for
x = 0.20; a further increase of the doping concentration decreases the conductivity. The oxygen vacancies inside the grain and grain boundary control the whole conduction process. This variation may be due to the number of oxygen vacancies present in the system and the interaction between the oxygen vacancies and dopant cations. As discussed earlier, the oxygen vacancies increase with doping concentration; therefore, conductivity is expected to increase with doping concentration. At higher doping concentrations, the number of oxygen vacancies will be greater, so there may be strong interactions between these vacancies and Dy
3+ ions. Therefore, the motion of free oxygen vacancies at higher doping concentrations should be limited, and conductivity decreases.
Fig. 8(c) shows the temperature dependence of DC conductivity
σ(0), which obeys the Arrhenius equation given by
| |
 | (10) |
where
σ0 is the pre-exponential factor, being a constant in a certain temperature range,
KB is Boltzmann's constant,
T is the absolute temperature and
Ea is the activation energy for DC conduction.
Ea can be calculated easily from the slope of the Arrhenius plot. It is observed in
Fig. 8(c) that the DC conductivity increases with temperature, which indicates that oxygen ion conduction in these compositions is a thermally activated process. The activation energies for DC conduction are listed in
Table 4. The activation energy shows a minimum value for the sample
x = 0.20, which shows the maximum conductivity. In another study by S. Kuharuangrong,
53 the activation energies for
x = 0.10,
x = 0.20 and
x = 0.30 were reported as 1.06 eV, 1.11 eV and 1.15 eV respectively, and the composition
x = 0.30 showed the highest conductivity among all the compositions. These activation energy values are very close to those found for our system. Again, according to S. Acharya,
15 the composition
x = 0.15 shows the lowest activation energy (0.86 eV) and the highest conductivity (7.42 × 10
−2 S cm
−1). A. K. Baral
et al.27 had reported the activation energy and conductivity at 550 °C for the composition
x = 0.20 in their study as 1.16 eV and 1.36 × 10
−4 S cm
−1, respectively. However, in our present system, the composition
x = 0.20 shows a lower activation energy and higher conductivity at 550 °C (5 × 10
−4 S cm
−1) than the previous study by A. K. Baral
et al. Again, Y. Wang
et al.54 reported the activation energy and conductivity for the composition
x = 0.10 in their study as 0.79 eV and 2 × 10
−4 S cm
−1, respectively. Therefore, our conductivity and activation energies are comparable to previously reported studies. The activation energy and conductivity values of our present study and previous studies are also listed in
Table 4. The scaling behavior of the conductivity spectra shows the effect of temperature on the conduction mechanism. The conductivity spectra of the compositions obey the time–temperature superposition principle (TTSP),
i.e. all the conductivity spectra at different temperatures are superimposed on a single master curve. In the case of the real part of the complex conductivity, the TTSP can be represented by the following scaling law:
55| |
 | (11) |
Table 4 The values of activation energy (Ea) and conductivities for the samples Ce1−xDyxO2−δ (x = 0.10–0.50). The errors after the second decimal are indicated inside parentheses
| Sample |
Activation energy (eV) |
Conductivity at 550 °C (Ω−1 cm−1) |
| Ce0.9Dy0.1O2−δ |
1.12(2) |
5 × 10−5 |
| 0.79 (ref. 54) |
2 × 10−4 |
| Ce0.85Dy0.15O2−δ |
1.10(9) |
3 × 10−4 |
| 0.86 (ref. 15) |
7.42 × 10−2 |
| Ce0.8Dy0.2O2−δ |
1.10(5) |
5 × 10−4 |
| 1.16 (ref. 27) |
1.36 × 10−4 |
| Ce0.75Dy0.25O2−δ |
1.15(8) |
3.3 × 10−4 |
| Ce0.7Dy0.3O2−δ |
1.19(8) |
2.9 × 10−4 |
| 1.15 (ref. 53) |
| Ce0.6Dy0.4O2−δ |
1.21(4) |
8 × 10−5 |
| Ce0.5Dy0.5O2−δ |
1.46(2) |
1 × 10−5 |
The scaling function F is independent of both the temperature and composition. Fig. 9(a) shows the superposition of all the curves on a master curve for x = 0.20. Similar behavior was also found for other compositions. This behavior simply indicates that the conduction mechanism (movement of oxygen ions through the crystal lattice as a result of thermally activated hopping of the oxygen ions moving from a crystal lattice site to another crystal lattice site) does not depend on temperature, i.e. the change in temperature only changes the number of charge carriers without changing the conduction mechanism.56 Fig. 9(b) shows the scaled spectra for different compositions for a particular temperature, which shows the superposition of all the curves on a single curve. This indicates the doping concentration independence of the conduction mechanism. More clearly, the migration of charge carriers in our present system takes place through a simple hopping mechanism. The only likely migration events involve an oxygen ion hopping to a vacancy in a nearest neighbor or a next nearest neighbor site, thus exchanging its place with the vacancy. The increase of the doping concentration only changes the number of oxygen vacancies; the conduction mechanism, as mentioned above, remains unchanged.
 |
| | Fig. 9 (a) Scaling of the real part of the ac conductivity σ′(ω) of the complex conductivity spectra σ*(ω) for Ce0.8Dy0.2O2−δ at several temperatures and (b) conductivity master curve for different compositions at a temperature of 500 °C. | |
4. Conclusion
In summary, Dy doped ceria-based nanoparticles were obtained by the citrate auto-ignition method. The good stoichiometry of the atoms in the obtained samples was confirmed by EDAX. Rietveld analysis of the XRD data and HRTEM of the sintered samples confirmed their good crystallinity and single phase cubic fluorite structures with space group Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. The variation of the lattice parameter with doping concentration tends to saturate at higher doping concentrations due to interactions between the oxygen vacancies and cations. The UV-VIS absorption measurements showed a red shift in the absorption peak positions with the concentration of Dy3+ ions, and were explained in terms of the compensation of Ce3+ ions, generation of oxygen vacancies and creation of f-energy states. The Rietveld analysis and the shift of the different Raman bands confirmed the increase of oxygen vacancies with doping concentration. The Raman spectra also reveal the presence of different defect spaces, such as M4Ov, O6Ov, Ce3+O8 and Dy3+O8 type complexes, including oxygen vacancies. The DC conductivity and activation energy vary with doping concentration due to oxygen vacancies and to interactions between the vacancies and the dopant cations.
m. The variation of the lattice parameter with doping concentration tends to saturate at higher doping concentrations due to interactions between the oxygen vacancies and cations. The UV-VIS absorption measurements showed a red shift in the absorption peak positions with the concentration of Dy3+ ions, and were explained in terms of the compensation of Ce3+ ions, generation of oxygen vacancies and creation of f-energy states. The Rietveld analysis and the shift of the different Raman bands confirmed the increase of oxygen vacancies with doping concentration. The Raman spectra also reveal the presence of different defect spaces, such as M4Ov, O6Ov, Ce3+O8 and Dy3+O8 type complexes, including oxygen vacancies. The DC conductivity and activation energy vary with doping concentration due to oxygen vacancies and to interactions between the vacancies and the dopant cations.
Acknowledgements
One of the authors (AD) thankfully acknowledges the financial assistance from Department of Science and Technology (Govt. of India) (Grant no. SR/FTP/PS-141-2010). The authors (SA and AD) also acknowledge the instrumental support from DST (Govt. of India) under departmental FIST programme (Grant no. SR/FST/PS-II-001/2011) and University Grants Commission (UGC) for departmental CAS scheme.
Reference
- B. C. H. Steele, Solid State Ionics, 2000, 129, 95–110 CrossRef CAS.
- E. Bekyarova, P. Fornasiero, J. Kaspar and M. Graziani, Catal. Today, 1998, 45, 179–183 CrossRef CAS.
- L. Zhaogang, L. Mei, H. Yanhong, W. Mitang and S. Zhenxue, J. Rare Earths, 2008, 26, 158–162 CrossRef.
- Z.-P. Li, T. Mori, F. Ye, D. Ou, J. Zou and J. Drennam, J. Chem. Phys., 2011, 134, 224708 CrossRef PubMed.
- B. Choudhury and A. Choudhury, Curr. Appl. Phys., 2013, 13, 217–223 CrossRef PubMed.
- J. Ueda and S. Tanabe, J. Appl. Phys., 2011, 110, 073104 CrossRef PubMed.
- M. Yashima, S. Kobayashi and T. Yasui, Faraday Discuss., 2007, 134, 369–376 RSC.
- J. M. de Souza e Silva, M. Strauss, C. M. Maroneze, E. R. Souza, Y. Gushikem, F. A. Sigoli and I. O. Mazali, J. Mater. Chem., 2011, 21, 15678–15685 RSC.
- Z. P. Li, T. Mori, F. Ye, D. Ou, G. Auchterlonie, J. Zou and J. Drennan, J. Phys. Chem. C, 2012, 116, 5435–5443 CAS.
- Z. P. Li, T. Mori, J. Zou and J. Drennan, Mater. Res. Bull., 2013, 48, 807–812 CrossRef CAS PubMed.
- M. Greenberg, E. Wachtel, I. Lubomirsky, J. Fleig and J. Maier, Adv. Funct. Mater., 2006, 16, 48–52 CrossRef CAS PubMed.
- T. Taniguchi, T. Watanabe, N. Sugiyama, A. K. Subramani, H. Wagata, N. Matsushita and M. Yoshimura, J. Phys. Chem. C, 2009, 113, 19789–19793 CAS.
- G. B. Balazs and R. S. Glass, Solid State Ionics, 1995, 76, 155–162 CrossRef.
- H. Inaba and H. Tagawa, Solid State Ionics, 1996, 83, 1–16 CrossRef CAS.
- S. A. Acharya, J. Power Sources, 2011, 198, 105–111 CrossRef PubMed.
- S. Anirban and A. Dutta, J. Phys. Chem. Solids, 2015, 76, 178–183 CrossRef CAS PubMed.
- S. Sain, S. Patra and S. K. Pradhan, Mater. Res. Bull., 2012, 47, 1062–1072 CrossRef CAS PubMed.
- H. M. Rietveld, J. Appl. Crystallogr., 1969, 2, 65–71 CrossRef CAS.
- L. Lutterolti, P. Scardi and P. Maistrelli, J. Appl. Crystallogr., 1992, 25, 459–462 CrossRef.
- R. A. Young in, The Rietveld method, ed, R. A. Young, Oxford University Press/IUCr, 1996, pp. 1–38 Search PubMed.
- L. B. McCusker, R. B. Von Dreele, D. E. Cox, D. Louer and P. Scardi, J. Appl. Crystallogr., 1999, 32, 36–50 CrossRef CAS.
- N. C. Popa, J. Appl. Crystallogr., 1998, 31, 176–180 CrossRef CAS.
- S. Gangopadhyay, D. D. Frolov, A. E. Masunov and S. Seal, J. Alloys Compd., 2014, 584, 199–208 CrossRef CAS PubMed.
- M. Nakayama and M. Martin, Phys. Chem. Chem. Phys., 2009, 11, 3241–3249 RSC.
- S. J. Hong, K. Mehta and A. V. Virkar, J. Electrochem. Soc., 1998, 145, 638–647 CrossRef CAS PubMed.
- T. Mori, T. Kobayashi, Y. Wang, J. Drennan, T. Nishimura, J. G. Li and H. Kobayashi, J. Am. Ceram. Soc., 2005, 88, 1981–1984 CrossRef CAS PubMed.
- A. K. Baral and V. Sankaranarayanan, Nanoscale Res. Lett., 2010, 5, 637–643 CrossRef CAS PubMed.
- L. Vegard and H. Dale, Z. Kristallogr., 1928, 67, 148–162 CAS.
- D. Pérez-Coll, P. Núñ ez, J. C. Ruiz-Morales, J. Peñ a-Martíez and J. R. Frade, Electrochim. Acta, 2007, 52, 2001–2008 CrossRef PubMed.
- S. Omar, E. D. Wachsman and J. C. Nino, Solid State Ionics, 2008, 178, 1890–1897 CrossRef CAS PubMed.
- L. Li, G. Li, Y. Che and W. Su, Chem. Mater., 2000, 12, 2567–2574 CrossRef CAS.
- D. R. Ou, T. Mori, F. Ye, J. Zou, G. Auchterlonie and J. Drennan, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 024108 CrossRef.
- Y.-W. Zhang, R. Si, C.-S. Liao, C.-H. Yan, C.-X. Xiao and Y. Kou, J. Phys. Chem. B, 2003, 107, 10159–10167 CrossRef CAS.
- S. L. S. Rao, G. Ramdevudu, Md. Shareefuddin, A. Hameed, M. N. Chary and M. L. Ro, Int. J. Eng. Sci. Res. Technol., 2012, 4, 25–35 Search PubMed.
- E. Wuilloud, B. Delley, W. D. Schneider and Y. Baer, Phys. Rev. Lett., 1984, 53, 202–205 CrossRef CAS.
- L. Wu, H. J. Wiesmann, A. R. Moodenbaugh, R. F. Klie, Y. Zhu, D. O. Welch and M. Suenaya, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 69, 125415 CrossRef.
- G.-R. Li, D.-L. Qu, L. Arurault and Ye-X. Tong, J. Phys. Chem. C, 2009, 113, 1235–1241 CAS.
- K. Kuntaiah, P. Sudarsanam, B. M. Reddy and A. Vinu, RSC Adv., 2013, 3, 7953–7962 RSC.
- Z. Wang, Z. Quan and J. Lin, Inorg. Chem., 2007, 46, 5237–5242 CrossRef CAS PubMed.
- C. Sun, H. Li, H. Zhang, Z. Wang and L. Chen, Nanotechnology, 2005, 16, 1454–1463 CrossRef CAS.
- A. H. Morshed, M. E. Moussa, S. M. Bedair, R. Leonard, S. X. Liu and N. El-Masry, Appl. Phys. Lett., 1997, 70, 1647–1649 CrossRef CAS PubMed.
- M. Sarkar, R. Rajkumar, S. Tripathy and S. Balakumar, Mater. Res. Bull., 2012, 47, 4340–4346 CrossRef PubMed.
- I. Atribak, A. Bueno-López and A. García- García, J. Catal., 2008, 259, 123–132 CrossRef CAS PubMed.
- J. R. McBride, K. C. Hass, B. D. Poindexter and W. H. Weber, J. Appl. Phys., 1994, 76, 2435–2441 CrossRef CAS PubMed.
- E. C. C. Souza and E. N. S. Muccillo, J. Alloys Compd., 2009, 473, 560–566 CrossRef CAS PubMed.
- T. Taniguchi, T. Watanabe, N. Sugiyama, A. K. Subramani, H. Wagata, N. Matsushita and M. Yoshimura, J. Phys. Chem. C, 2009, 113, 19789–19793 CAS.
- R. O. da Fonseca, A. A. A. da Silva, M. R. M. Signorelli, R. C. Rabelo-Neto, F. B. Noronha, R. C. C. Simões and L. V. Mattos, J. Braz. Chem. Soc., 2014, 25, 2356–2363 Search PubMed.
- A. Nakajima, A. Yoshihara and M. Ishigame, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 13297–13307 CrossRef CAS.
- S. F. Wang, C. T. Yeh, Y. R. Wang and Y. C. Wu, J. Mater. Res. Technol., 2013, 2, 141–148 CrossRef CAS PubMed.
- Z. D. Dohčević-Mitrović, M. J. Šćepanović, M. U. Grujić-Brojčin, Z. V. Popović, S. B. Bošković, B. M. Matovic, M. V. Zinkevich and F. Aldinger, Solid State Commun., 2006, 137, 387–390 CrossRef PubMed.
- Raghvendra, R. K. Singh and P. Singh, J. Mater. Sci., 2014, 49, 5571–5578 CrossRef CAS.
- J. C. Dyre, J. Appl. Phys., 1988, 64, 2456–2468 CrossRef PubMed.
- S. Kuharuangrong, J. Power Sources, 2007, 171, 506–510 CrossRef CAS PubMed.
- Y. Wang, T. Mori, J. G. Li and J. Drennan, J. Eur. Ceram. Soc., 2005, 25, 949–956 CrossRef CAS PubMed.
- B. Roling, A. Happe, K. Funke and M. D. Ingram, Phys. Rev. Lett., 1997, 78, 2160 CrossRef CAS.
- Raghvendra, P. Singh and R. K. Singh, J. Alloys Compd., 2013, 549, 238–244 CrossRef CAS PubMed.
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m of all the samples. The EDAX spectra show good stoichiometry of the different atoms in the samples. The direct band gap, calculated from UV-vis spectra, shows a red shift with increasing concentration of Dy3+ ions. Raman spectroscopy studies of the samples gave insight into their vibrational properties and pointed to the fact that the oxygen vacancy content increased significantly with the doping concentration. The number of oxygen vacancies and their interaction with dopant cations strongly influence the electrical properties of Dy doped ceria.
m of all the samples. The EDAX spectra show good stoichiometry of the different atoms in the samples. The direct band gap, calculated from UV-vis spectra, shows a red shift with increasing concentration of Dy3+ ions. Raman spectroscopy studies of the samples gave insight into their vibrational properties and pointed to the fact that the oxygen vacancy content increased significantly with the doping concentration. The number of oxygen vacancies and their interaction with dopant cations strongly influence the electrical properties of Dy doped ceria.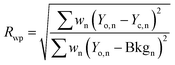
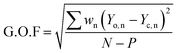
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. In this structure, cerium ions occupy the vertices and face of the cubic unit cell. Each cerium ion (Ce4+) is co-ordinated with eight oxygen ions (O2−) arranged in a perfect cube, while each oxygen ion is surrounded by four cerium ions in a tetrahedral arrangement. This structure is often described as a cubic closed packing of cerium ions with oxygen ions occupying all tetrahedral holes.23 The crystal structure of pure ceria is shown in Fig. 2(a). All the cerium ions (Ce4+) are situated at site 4a with the atomic co-ordinate (0,0,0), and the oxygen ions (O2−) are at site 8c, corresponding to the (1/4, 1/4, 1/4) position. All the ion positions are fixed during the whole refinement process. Generally, when the fluorite structured ceria is doped with a trivalent cation such as Dy3+, one oxygen vacancy is formed for every two trivalent cations for charge neutrality, which is represented by Kröger–Vink notation:
m. In this structure, cerium ions occupy the vertices and face of the cubic unit cell. Each cerium ion (Ce4+) is co-ordinated with eight oxygen ions (O2−) arranged in a perfect cube, while each oxygen ion is surrounded by four cerium ions in a tetrahedral arrangement. This structure is often described as a cubic closed packing of cerium ions with oxygen ions occupying all tetrahedral holes.23 The crystal structure of pure ceria is shown in Fig. 2(a). All the cerium ions (Ce4+) are situated at site 4a with the atomic co-ordinate (0,0,0), and the oxygen ions (O2−) are at site 8c, corresponding to the (1/4, 1/4, 1/4) position. All the ion positions are fixed during the whole refinement process. Generally, when the fluorite structured ceria is doped with a trivalent cation such as Dy3+, one oxygen vacancy is formed for every two trivalent cations for charge neutrality, which is represented by Kröger–Vink notation: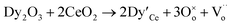
 represents the oxygen vacancy. The defect structure of the doped ceria is shown in Fig. 2(b). Here, the occupancy of Ce4+ ions in the 4a site and O2− ions in the 8c site of undoped ceria are 0.9989(7) and 0.9859(1) respectively (Table 1). In doped ceria, the occupancy of Ce4+ ions decreases with the doping concentration, and the occupancy of dopant ions (Dy3+) in the 4a site increases. The occupancy of O−2 ions at the 8c site decreases with the doping concentration of Dy3+ ions, which indicates a higher number of oxygen vacancies. The formation of these oxygen vacancies has been confirmed in earlier studies.24–27 Fig. 3 shows the variation of the lattice parameters of the Ce1−xDyxO2−δ (x = 0.00–0.50) samples as a function of total dopant concentration (x). It can be seen that the lattice parameter increases with dopant concentration, which is due to the fact that the ionic radius of Dy3+ (1.027 Å) is greater than the ionic radius of Ce4+ (0.97 Å). It can be seen from Fig. 3 that for the Ce1−xDyxO2−δ system, the lattice parameter increases linearly with increasing Dy3+ concentration up to x = 0.25, following Vegard's law.28 After x = 0.25, the lattice parameter of the Ce1−xDyxO2−δ system also increases linearly, but the rate of increase is different. Using a least-squares fitting algorithm, a linear relationship was obtained between the lattice parameters (a) and dopant concentration (x). These can be represented as
represents the oxygen vacancy. The defect structure of the doped ceria is shown in Fig. 2(b). Here, the occupancy of Ce4+ ions in the 4a site and O2− ions in the 8c site of undoped ceria are 0.9989(7) and 0.9859(1) respectively (Table 1). In doped ceria, the occupancy of Ce4+ ions decreases with the doping concentration, and the occupancy of dopant ions (Dy3+) in the 4a site increases. The occupancy of O−2 ions at the 8c site decreases with the doping concentration of Dy3+ ions, which indicates a higher number of oxygen vacancies. The formation of these oxygen vacancies has been confirmed in earlier studies.24–27 Fig. 3 shows the variation of the lattice parameters of the Ce1−xDyxO2−δ (x = 0.00–0.50) samples as a function of total dopant concentration (x). It can be seen that the lattice parameter increases with dopant concentration, which is due to the fact that the ionic radius of Dy3+ (1.027 Å) is greater than the ionic radius of Ce4+ (0.97 Å). It can be seen from Fig. 3 that for the Ce1−xDyxO2−δ system, the lattice parameter increases linearly with increasing Dy3+ concentration up to x = 0.25, following Vegard's law.28 After x = 0.25, the lattice parameter of the Ce1−xDyxO2−δ system also increases linearly, but the rate of increase is different. Using a least-squares fitting algorithm, a linear relationship was obtained between the lattice parameters (a) and dopant concentration (x). These can be represented as


 and
and  complexes. Both the D1 and D2 bands shift towards higher wavenumbers with increasing concentration of Dy3+ ions, and the shift of D1 is assigned to the increase of the
complexes. Both the D1 and D2 bands shift towards higher wavenumbers with increasing concentration of Dy3+ ions, and the shift of D1 is assigned to the increase of the  complex compared to that of the
complex compared to that of the 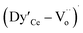 complex. The ratio of the intensities of the different bands is listed in Table 3. The ratio of IF2g/ID1 and IF2g/ID2 decreases with increasing x, which is related to the degree of defect sites on CeO2. This also indicates a higher number of oxygen vacancies with higher doping concentrations.47 According to Nakajima et al.,48 a low ratio of ID1/ID2 indicates that Ce3+ ions preferentially locate in a Ce3+O8 type complex, and a higher value of this ratio indicates an increase in the concentration of the amalgamated defects of O2− vacancies in Dy3+O8 type complexes. S. F. Wang et al.49 and Z. D. Dohčević-Mitrović et al.50 have also shown the increase of oxygen vacancies with doping concentration in rare earth doped ceria solid solutions using Raman spectroscopy. The shift of the Raman bands and the change in the intensity ratio with doping concentration also confirms the formation of solid solutions in our present study by the citrate auto-ignition method.
complex. The ratio of the intensities of the different bands is listed in Table 3. The ratio of IF2g/ID1 and IF2g/ID2 decreases with increasing x, which is related to the degree of defect sites on CeO2. This also indicates a higher number of oxygen vacancies with higher doping concentrations.47 According to Nakajima et al.,48 a low ratio of ID1/ID2 indicates that Ce3+ ions preferentially locate in a Ce3+O8 type complex, and a higher value of this ratio indicates an increase in the concentration of the amalgamated defects of O2− vacancies in Dy3+O8 type complexes. S. F. Wang et al.49 and Z. D. Dohčević-Mitrović et al.50 have also shown the increase of oxygen vacancies with doping concentration in rare earth doped ceria solid solutions using Raman spectroscopy. The shift of the Raman bands and the change in the intensity ratio with doping concentration also confirms the formation of solid solutions in our present study by the citrate auto-ignition method.

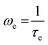 is the attempt frequency to overcome the highest free energy barrier. The real part of σ*(ω) has been used to fit the conductivity spectra, as shown in Fig. 8(a). The variation of σ(0) with the doping concentration of Dy3+ for different temperatures is shown in Fig. 8(b). This figure reveals that the DC conductivity increases with doping concentration and shows a maximum value for x = 0.20; a further increase of the doping concentration decreases the conductivity. The oxygen vacancies inside the grain and grain boundary control the whole conduction process. This variation may be due to the number of oxygen vacancies present in the system and the interaction between the oxygen vacancies and dopant cations. As discussed earlier, the oxygen vacancies increase with doping concentration; therefore, conductivity is expected to increase with doping concentration. At higher doping concentrations, the number of oxygen vacancies will be greater, so there may be strong interactions between these vacancies and Dy3+ ions. Therefore, the motion of free oxygen vacancies at higher doping concentrations should be limited, and conductivity decreases. Fig. 8(c) shows the temperature dependence of DC conductivity σ(0), which obeys the Arrhenius equation given by
is the attempt frequency to overcome the highest free energy barrier. The real part of σ*(ω) has been used to fit the conductivity spectra, as shown in Fig. 8(a). The variation of σ(0) with the doping concentration of Dy3+ for different temperatures is shown in Fig. 8(b). This figure reveals that the DC conductivity increases with doping concentration and shows a maximum value for x = 0.20; a further increase of the doping concentration decreases the conductivity. The oxygen vacancies inside the grain and grain boundary control the whole conduction process. This variation may be due to the number of oxygen vacancies present in the system and the interaction between the oxygen vacancies and dopant cations. As discussed earlier, the oxygen vacancies increase with doping concentration; therefore, conductivity is expected to increase with doping concentration. At higher doping concentrations, the number of oxygen vacancies will be greater, so there may be strong interactions between these vacancies and Dy3+ ions. Therefore, the motion of free oxygen vacancies at higher doping concentrations should be limited, and conductivity decreases. Fig. 8(c) shows the temperature dependence of DC conductivity σ(0), which obeys the Arrhenius equation given by

![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. The variation of the lattice parameter with doping concentration tends to saturate at higher doping concentrations due to interactions between the oxygen vacancies and cations. The UV-VIS absorption measurements showed a red shift in the absorption peak positions with the concentration of Dy3+ ions, and were explained in terms of the compensation of Ce3+ ions, generation of oxygen vacancies and creation of f-energy states. The Rietveld analysis and the shift of the different Raman bands confirmed the increase of oxygen vacancies with doping concentration. The Raman spectra also reveal the presence of different defect spaces, such as M4Ov, O6Ov, Ce3+O8 and Dy3+O8 type complexes, including oxygen vacancies. The DC conductivity and activation energy vary with doping concentration due to oxygen vacancies and to interactions between the vacancies and the dopant cations.
m. The variation of the lattice parameter with doping concentration tends to saturate at higher doping concentrations due to interactions between the oxygen vacancies and cations. The UV-VIS absorption measurements showed a red shift in the absorption peak positions with the concentration of Dy3+ ions, and were explained in terms of the compensation of Ce3+ ions, generation of oxygen vacancies and creation of f-energy states. The Rietveld analysis and the shift of the different Raman bands confirmed the increase of oxygen vacancies with doping concentration. The Raman spectra also reveal the presence of different defect spaces, such as M4Ov, O6Ov, Ce3+O8 and Dy3+O8 type complexes, including oxygen vacancies. The DC conductivity and activation energy vary with doping concentration due to oxygen vacancies and to interactions between the vacancies and the dopant cations.