Enhancing the electrode performance of Co3O4 through Co3O4@a-TiO2 core–shell microcubes with controllable pore size†
Yanting Chua,
Jinkui Fenga,
Yitai Qianab and
Shenglin Xiong*ac
aKey Laboratory of the Colloid and Interface Chemistry, Ministry of Education, School of Chemistry and Chemical Engineering, Shandong University, Jinan, 250100, P. R. China. E-mail: chexsl@sdu.edu.cn
bHefei National Laboratory for Physical Science at Microscale and Department of Chemistry, University of Science and Technology of China, Hefei, Anhui 230026, P. R. China
cCAS Key Laboratory of Materials for Energy Conversion, University of Science and Technology of China, Hefei, Anhui 230026, P. R. China
First published on 29th April 2015
Abstract
Co3O4 is a potential high-capacity anode material for lithium ion batteries (LIBs), but it usually has poor cycling stability due to its enormous volume variation during the conversion reaction process. A protection strategy has been developed to enhance the electrode performance of Co3O4 through construction of Co3O4@amorphous-TiO2 (Co3O4@a-TiO2) core–shell nanostructures with controllable pore size, realized by precisely regulating the volume ratio of ethanol to water. To our knowledge, this is the first time to use core–shell Co3O4@a-TiO2 as the anode material for the lithium-ion batteries. Noteworthy, electrochemical performances of the materials were found to be strongly correlated with their porous structures. With the excellent stability of TiO2 sheath, high capacity of Co3O4 core, and the optimized porous size, these unique Co3O4@a-TiO2 core–shell microcubes exhibit high capacity, long cycle life and good rate capability as advanced electrode materials for lithium-ion batteries. A high reversible capacity of ∼800 mA h g−1 is obtained at 500 mA g−1, keeping the value stable after 60 cycles. We anticipate that such a demonstration presents a versatile method toward synthesizing other high-performance and multifunctional metal oxides@a-TiO2 nanocomposites for LIBs in near future.
1. Introduction
Lithium-ion batteries (LIBs) are one of the most promising energy storage devices for their advantages, including long lifetime, high capacities and energy density. However, restricted by some disadvantages, the present electrode materials could not satisfy the requirements of high-energy applications such as portable electronics, electric vehicles (EVs) and hybrid electric vehicles (HEVs). Recently, transitional metal oxides (TMOs)-based nanostructured materials have been widely used as negative electrode materials for LIBs due to high specific capacity (Fe2O3, 1007 mA h g−1; MnO2, 1232 mA h g−1), which is two or three times higher than that of currently used graphite anode (≈370 mA h g−1). Cobalt oxides (mainly Co3O4 and CoO), among the alternative anode candidates available, have been judiciously selected due to low cost synthesis and their much higher capacity than that of conventional graphite.1–4 In the scenario, lithium storage is mainly relied on the reversible conversion between lithium ions and oxide anode accompanied by formation of metallic nanocrystals dispersing in Li2O matrix. During this process, capacity usually degrades in a fast rate caused by noticeable volume expansion and consequential destruction of the electrode. Moreover, the intrinsic drawback of low electronic conductivity also, to some extent, contributes to performance degradation in metal oxide involved electrodes, especially at high current densities. To circumvent the problems, development of cobalt oxide based micro/nanostructures engineered with optimized particle sizes, configuration and component compositions, had been made.5,6 Despite dramatic endeavors to exploit the field of anode materials, how to fabricate truly durable cobalt oxide electrodes remains a noteworthy challenge with desired rate capability and specific capacity.Recently, hybridization of nanostructures, consisting of several components, has been emerging as one of the most feasible means towards high-performance materials for LIBs. In this regard, different motifs are explored with active materials either embedded into or assembled onto the conductive matrix by virtue of chemical bonding or various noncovalent interactions. The point of such hybrid structures is to induce strong synergetic effect obtained via integrating individual components, not existing before hybridization, besides the previously-existent functions of each constituent. As a consequence, novel functionalities can be realized by the full potential of composite materials. In fact, this concept affords the versatility in no matter engineering or science fields, wherein recent achievements have been promoted such as Fe2O3/SnO2, Fe2O3/TiO2, Fe2O3/ZnO, TiO2/SnO2, MnO2/Fe2O3, and so on.7–18 Amongst them, titanium dioxide (TiO2) shows nice adaptability to different applications considering its intrinsic properties such as good chemical and physical stability, low cost, environmental friendliness, and plentiful polymorphs.19 Hence, the integration with TiO2 offers a promising possibility to improve the LIBs capacity and cycling stability. It should be mentioned that several polymorphs of TiO2, including rutile,20 anatase21 and brookite,22,23 and even amorphous,24 have been investigated for LIBs. Most reports focus on the study of abundant morphologies of anatase and rutile whereas few are pertain to amorphous TiO2.
Stimulated by the above considerations, herein, we proposed a convenient approach by combining solvothermal and sol–gel processes to construct 3D porous Co3O4@a-TiO2 core–shell micro/nanostructures. More importantly, by adjusting the solvent composition in the sol–gel step, the shell-TiO2 with controllable pore size was obtained (see Fig. 1). Such a novel hybrid micro/nanostructure is expected to confirm remarkably enhanced electrochemical performance due to the incorporation of favorable structural features. To be specific, excellent stability of TiO2 sheath, remarkable capacity of Co3O4 core, and the appropriate pore size distribution are favorable for the stability of electrode materials during the discharge–charge process. When served as the anode material for LIBs, 3D porous Co3O4@a-TiO2 core–shell architectures with optimal pore size indicate remarkable electrochemical performance. A high reversible capacity of ∼800 mA h g−1 was reached and kept stable at 500 mA g−1.
2. Experimental section
Synthesis of Co3O4 microcubes
All of the reagents were purchased and used without further purification. CoCO3 micro/nanoparticles were prepared according to our previous modified method with several variation.25 In a typical synthesis, 1.5 mmol of Co(CH3COO)2·4H2O and 30 mmol NH4HCO3 were dissolved in 40 mL HOC2H4OH under stirring. The as-obtained homogeneous mixed solution was transferred into 60 mL Teflon-lined stainless steel autoclave after stirring for 1 h and heated at 200 °C for 20 h. The obtained pink precipitation were washed for three times by a ethanol–water mixture, and dried in air at 80 °C overnight. The CoCO3 precursors were converted to black Co3O4 powers after annealing at 300 °C for 4 h in air.Synthesis of Co3O4@a-TiO2 composites
Typically, about 0.1 g of the Co3O4 microcubes were well dispersed in ethanol (40 mL) and deionized water (0.3 mL) under stirring for 30 min and ultrasonication for 15 min at room temperature. After adding 0.5 mL tetrabutyl orthotitanate (TBOT) in ethanol (3 mL) dropwise, the solution was heated to 80 °C in an oil bath for 100 min. The product was collected through centrifugation and washed with ethanol–water mixture for several times. Eventually, the collection was dried in a vacuum oven at 80 °C. In order to obtain the other two products, we can alter the volume of the ethanol with the other experiment conditions unchanged.Materials characterization
SEM images were obtained with a field-emission scanning electron microscope (FESEM, JSM-6700F, JEOL) operated at 5 kV. High-resolution TEM (HRTEM), scanning TEM, and energy dispersive X-ray (EDX) analysis were collected on a JEM-2100F (JEOL) instruments operating at 200 kV accelerating voltage. X-Ray photoelectron spectra (XPS) measurements were conducted with an X-ray photoelectron spectroscopy (XPS, VGESCA-LABMK II spectrometer, (ESCALAB 250) using a twin-anode Al Kα (1486.6 eV) X-ray source). Power X-ray diffraction (PXRD) characterization was carried out using a Bruker-D8 Advanced X-ray diffractometer with a Cu Kα radiation source and a wavelength of 1.5406 Å. The N2 adsorption–desorption isotherms were obtained from a Micromeritics analyzer (ASAP 2020, USA) at 77 K.Electrochemical measurements
Electrochemical experiments were performed in 2032 coin type cells. The working electrodes were prepared by mixing the samples (Co3O4 or Co3O4@a-TiO2), acetylene black, and polymer binder (sodium carboxymethyl cellulose, CMC) at a weight ratio of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and pasted on pure copper foil and the thickness of the coating is 200 μm. After drying under vacuum at 80 °C for 12 h, the resulting foil was roll-pressed and cut into 12 mm discs in diameter, making the density of the active material is about 1 mg cm−2. Lithium foil was used as both the counter electrode and reference electrode, and the Celgard 2300 microporous polypropylene membrane was used as the separator. The electrolyte consisting of a solution of 1 M LiPF6 in ethylene carbonate (EC), dimethylcarbonate (DMC) and diethyl carbonate (DEC) (1
10 and pasted on pure copper foil and the thickness of the coating is 200 μm. After drying under vacuum at 80 °C for 12 h, the resulting foil was roll-pressed and cut into 12 mm discs in diameter, making the density of the active material is about 1 mg cm−2. Lithium foil was used as both the counter electrode and reference electrode, and the Celgard 2300 microporous polypropylene membrane was used as the separator. The electrolyte consisting of a solution of 1 M LiPF6 in ethylene carbonate (EC), dimethylcarbonate (DMC) and diethyl carbonate (DEC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v/v) was obtained from Samsung Chemical Ltd. The cells were assembled in an argon-filled glovebox (Mikrouna, Super 1220/750/900) with both the moisture and the oxygen content below 1 ppm. The galvanostatic charge–discharge measurements were performed using CT2001A LAND Cell test system at various current densities. The cyclic voltammetry (CV) was conducted at 0.1 mV s−1 with the range of 0.01–3.5 V on a LK2005A electrochemical workstation.
1, v/v/v) was obtained from Samsung Chemical Ltd. The cells were assembled in an argon-filled glovebox (Mikrouna, Super 1220/750/900) with both the moisture and the oxygen content below 1 ppm. The galvanostatic charge–discharge measurements were performed using CT2001A LAND Cell test system at various current densities. The cyclic voltammetry (CV) was conducted at 0.1 mV s−1 with the range of 0.01–3.5 V on a LK2005A electrochemical workstation.
3. Results and discussion
Uniform Co3O4 microcubes (noted as S-0) synthesized through a simple solvothermal and subsequent calcination are employed as the cores. As revealed by FESEM, these Co3O4 microcubes are uniform with an average diameter at ∼1 μm (Fig. 2a). Then, through a sol–gel process and adjusting the volume of the solvent, 3D core–shell Co3O4@a-TiO2 microcomposites with controllable pore size were synthesized (details see Experimental section), as displayed in Fig. 2b–d. When the solvent is 40 mL, the samples (noted as S-40) have the rough surfaces and consists of the small and tight nanoparticles (see Fig. 2b). As the volume of the ethanol increasing to 80 mL, the bigger and less tight a-TiO2 units were around the core Co3O4, forming another Co3O4@a-TiO2 samples (noted as S-80) (Fig. 2c). As shown in Fig. 2d, the surface a-TiO2 particles of the S-120 samples are the biggest and loosest, indicating the biggest pore size. The formation of the a-TiO2 layer is also supported by high-magnification TEM images. As described in Fig. S1,† the S-40, S-80 and S-120 show the a-TiO2 layer thickness of 73, 48 and 32 nm.On the basis of the formation mechanisms of Zhao's group,26 we propose a approximate coating process. When the addition of a small volume (40 mL) of ethanol into the system, the concentration of the titanium oligomers is high, resulting in making a-TiO2 shells in thickness. Upon increasing the volume of ethanol to 80 mL, making a lower concentration of the titanium oligomers, a thin a-TiO2 layers were formed on the surface of the Co3O4 nanoparticles. Finally, the thinnest a-TiO2 networks are formed. With a high volume of ethanol (120 mL), the concentration of the titanium oligomers is very low; thus there is only a very small amount of a-TiO2 coated on Co3O4. The formation process is indicated schematically in Fig. 1.
The morphology feature can further be elucidated from representative TEM image that the diameter of the cubic like S-0 is around 1 μm, as indicated in Fig. 3a. Low-magnification TEM image of the S-80 described in Fig. 3b shows that a-TiO2 nanoparticles are strongly anchored on the Co3O4, implying the formation of the Co3O4@a-TiO2. Fig. 3d indicates a locally magnified HRTEM image of core Co3O4 recorded from Fig. 3c. The 0.24 nm can be assigned to the (311) and (3−1−1) interplane spacings with zone axis of [0−11] parallel to incident electron direction. Conversely, no evident lattice fringes can be detected in Fig. 3c (the edge of the S-80 shown by white arrows), revealing that Co3O4 cores are well crystallized and TiO2 shells are amorphous. To indicate the phase structure of the samples, X-ray diffraction (XRD) measurements were performed. Fig. 3e presents XRD pattern of the S-0, S-40. The diffraction peaks of S-0 prepared by the pyrolysis of CoCO3, within the experimental error, can be assigned to cubic Co3O4 (a0 = 8.056 Å; space group Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (227), JCPDS Card no. 65-3103). Compared with the peaks of S-0, there are no difference in the pattern of S-40, indicating the presence of a-TiO2. In order to further illustrate the phase of the shell TiO2, the sample prepared without Co3O4 were tested. As shown in Fig. S2,† no crystal-clear diffraction peaks corresponding to the crystalline phase are detected, further revealing the amorphous structure of TiO2 in the three samples (S-40, S-80, S-120). Energy dispersive X-ray spectroscopy (EDS) maps clearly showed the distribution of Co, Ti, and O are quite uniform throughout the whole S-80, which demonstrates the formation of the a-TiO2.
m (227), JCPDS Card no. 65-3103). Compared with the peaks of S-0, there are no difference in the pattern of S-40, indicating the presence of a-TiO2. In order to further illustrate the phase of the shell TiO2, the sample prepared without Co3O4 were tested. As shown in Fig. S2,† no crystal-clear diffraction peaks corresponding to the crystalline phase are detected, further revealing the amorphous structure of TiO2 in the three samples (S-40, S-80, S-120). Energy dispersive X-ray spectroscopy (EDS) maps clearly showed the distribution of Co, Ti, and O are quite uniform throughout the whole S-80, which demonstrates the formation of the a-TiO2.
 | ||
| Fig. 3 (a) TEM of S-0. (b–d) TEM and HRTEM of S-80. (e) STEM image of S-80 and the corresponding elemental mappings for Co, Ti, O. (f) XRD pattern of the S-0, S-40. | ||
EDS presents the element analysis of the S-40, S-80, S-120 (Fig. S3†) and Co, Ti and O elements are detected in the three samples, indicating the coexistence of Co3O4 and a-TiO2. The Co/Ti ratio is further analyzed and confirmed by ICP-AES (Table S1†). The results further show the presence of the Co and Ti elements in the S-40, S-80, S-120. To further investigate the more detail elemental composition and the oxidation state of the Co3O4@a-TiO2 microcubes (S-40), we also carried out an X-ray photoelectron spectroscopy (XPS) measurements and the corresponding results are shown in Fig. 4. All of the binding energies (BEs) in this XPS analysis were corrected for specimen charging by referencing them to the C 1s peak (set at 284.6 eV). The survey spectrum (Fig. 4a) indicates the presence of the Co, Ti and O as well as C from the reference and the absence of other impurities, which is in good agreement with the EDS results. The high-resolution spectrum for the O 1s region (Fig. 4b) shows three oxygen contributions. The peaks at 529.2 eV and 530.0 eV are commonly assigned to the oxygen species of the Co–O and Ti–O bonds in the Co3O4 and TiO2.27–30 The peak sitting at 531.7 eV is usually ascribed to defects contaminants, and numerous surface species including hydroxyls, the oxygen in the physi- and chemi-sorbed water or under coordinated lattice.31–33 The Co 2p spectrum (Fig. 4c) indicates two major peaks with BE values at 779.6 and 794.7 eV, attributed to the Co 2p3/2 and Co 2p1/2 peaks, respectively, with a spin–orbit splitting of 15.1 eV.34,35 The absence of prominent shake-up satellite peaks in the Co 2p spectra further demonstrates the formation of the Co3O4 phase.2,36,37 The Ti 2p spectrum in Fig. 4d features two main spin–orbit lines of Ti 2p1/2 at 464.5 eV and Ti 2p3/2 at 458.6 eV, indicating the dominant Ti(IV). Furthermore, a peak separation of 5.9 eV is noticed between the two Ti 2p peaks, which is in good agreement with values reported in the literature.38 It is reasonable, therefore, to confirm that the composites consist of the core Co3O4 and the shell a-TiO2.
 | ||
| Fig. 4 XPS spectra of (a) survey spectrum, (b) O 1s, (c) Co 2p, and (d) Ti 2p for Co3O4@a-TiO2 (S-40). | ||
In order to verify the porous structure and the Brunauer–Emmett–Teller (BET) surface areas, the four samples are examined by N2 sorption at 77 K (Fig. 5). As shown in the Fig. 5a, a distinct hysteresis loop can be classified as type IV with a type H1 hysteresis loop, indicating the mesoporous structures. According to the corresponding Barrett–Joyner–Halenda (BJH) plots (Fig. 5b) recorded from nitrogen sorption of the S-0, S-40, S-80 and S-120 samples, the pore size distribution is in the 2–50 nm range and shows the domination of 14.8, 12.6, 11.6, 16.9 nm mesopores, respectively. Surface areas calculated by BET method are shown in Table S2,† indicating that specific surface areas were around 73.55, 105.67, 85.40, 56.38 m2 g−1 for S-0, S-40, S-80 and S-120 samples, respectively. While the volume of the ethanol is increased from 40 to 120 mL, the average pore size of the three Co3O4@a-TiO2 composites can be regulated from 5.2 to 10.87 nm, which is consistent with the observation of FESEM. Accordingly, the pore volumes of the Co3O4@a-TiO2 composites can also be regulated in the range from 0.1397 to 0.2088 cm3 g−1. The average pore size and pore volume of the S-0 samples are 9.49 nm and 0.2486 cm3 g−1, respectively.
 | ||
| Fig. 5 Nitrogen adsorption–desorption isotherms for the four samples (a) and the corresponding pore size distributions (b). | ||
Fig. 6a depicts the first five cyclic voltammetry (CV) curves of the S-80 electrodes between 0.01–3.5 V (vs. Li/Li+) with a scan rate of 0.1 mV s−1. In the first cathodic scan, the cathodic peak at 1.0 V was observed, which correspond to the insertion process of the Li+ in the a-TiO2. It can be clarified as follows: TiO2 + xLi+ + xe− → LixTiO2. The strong peak at around 0.8 V is assigned to the formation of a solid electrolyte interface (SEI) layer and the reduction of Co3O4,1,39 which can be described by Co3O4 +4Li+ + 4e− → Co + 2Li2O. There are two broad oxidation peaks at 1.3 V and 2.1 V in the first anodic process. The peak at 1.3 V usually is ascribed to the decomposition of the SEI layer and the peak at 2.1 V is allocated to the deinsertion of Li+ in the LixTiO2 and oxidation of Co to Co3O4. The second and onward CV curves remain overlapped, showing the excellent cycle stability of the S-80. The CV measurement is carried out to evaluated the electrochemical behavior of the Co3O4, as shown in Fig. S4,† which corresponds to the redox of Co/Co3O4 and the formation/decomposition of the SEI.
The electrochemical performance of the S-80 microcubes have been further investigated by galvanostatic discharge–charge tests at 0.5 A g−1 between 0.01 and 3.5 V, which is also consistent with that obtained from CV measurements, as shown in Fig. 6b. The voltage profiles of Co3O4@a-TiO2 composites (S-80) in the first cycle presented a slope between 1.7–0.9 V, which should be according to the amorphous TiO2. An inclined plateau between 0.9–0.7 V associated with the conversion from Co3O4 to an intermediate-phase LixCo3O4 and then to Co metal, followed by a sloping curve down to the voltage of 0.01 V, which is typical for the Co3O4 electrode. It is observed that the initial discharge and charge capacities are 1100 and 790 mA h g−1 for the Co3O4@a-TiO2 electrode, the initial capacity loss may result from the insufficient conversion reaction and irreversible lithium loss because of the formation of solid electrolyte interphase (SEI) film. The Coulombic efficiency (CE) of Co3O4@a-TiO2 composite rapidly rises to about 100% in the second one and then remains in the following cycles, showing an excellent reversibility.
To highlight the superiority of the S-80 for anode material of LIBs, the cycle performance of the S-80 was tested at a current 0.5 A g−1 for 60 cycles in the voltage of 0.01 to 3.5 V. For comparison, S-0, S-40, S-120 were also investigated under the same conditions. It is found that the capacity of the S-0 decays with cycling, and S-120 also shows a decreasing capacity when the capacity reaches its maximum value (Fig. 6c). It is supposed that no or too small amount of a-TiO2 restrict the cycling stability. The reversible capacities of S-40 and S-80 remain stable during 60 cycles. The capacity of the S-80 electrode, 800 mA h g−1, is the highest after 60 cycles, which can be attributed to the suitable amount of a-TiO2 and higher specific surface area. As expected, the S-80 electrode also exhibits good rate capacity, as displayed in Fig. 6d. S-40 displays the relatively lower specific discharge capacity at the current densities of 0.1–2 A g−1. The results show that too many a-TiO2 cause a decrease of the capacity. The corresponding CE of the cycling curves of four samples were supplied, as shown in Fig. S5.† For examples, their CE of S-80 quickly increases from 65% for the first cycle to about 99% after three cycles and remains nearly 100% thereafter, which reveals a convenient lithium insertion/extraction associated with efficient transport of ions and electrons in the electrodes. To further confirm the long cycling stability of the S-80 at higher current rate, the electrode was conducted at 2 A g−1 for 50 cycles as shown in Fig. S6.† It is noteworthy that the discharge capacity can retain a value as high as 400 mA h g−1 after more than 50 cycles, which is still much higher than the theoretical capacity of graphite (370 mA h g−1). To further understand the structural stability of the Co3O4@a-TiO2 composites (S-80), we studied the SEM and TEM after 20 discharge–charge cycles at 0.5 A g−1 (see the Fig. 7). As seen from the SEM and TEM images, some Co3O4@a-TiO2 porous microcubes still can be evident, suggesting its desirable structural stability over cycling, which could contribute greatly to the good cycling stability. For comparison, the cycling performance of pure a-TiO2 nanoparticles were tested, as shown in Fig. S7.† The reversible capacity of less than 200 mA h g−1 after the first cycle was sustained, confirming that TiO2 nanoparticles contributed very little to the capacity. It is noteworthy that the CE is very close to 100% all over the 200 cycles. These results suggest the a-TiO2 maintains extraordinarily stability and is in favor of the improvement of electrochemical performance of our composite. Thus the electrochemical performance of the materials can be effectively controlled by the pore size and the amount of the a-TiO2.
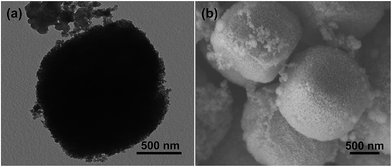 | ||
| Fig. 7 TEM (a) and SEM (b) images of the S-80 electrode after 20 charge–discharge cycles at current of 0.5 A g−1. | ||
4. Conclusion
In conclusion, an advanced Co3O4@amorphous-TiO2 (Co3O4@a-TiO2) core–shell nanostructures have been constructed through combining solvothermal and subsequent sol–gel processes. More importantly, through adjusting the solvent composition in the sol–gel step, the shell-TiO2 with controllable pore size was obtained. The as-obtained heterostructures combine both merits from TiO2 such as good cycle stability and Co3O4 with high capacity. With the excellent stability of TiO2 sheath, high capacity of Co3O4 core, the optimized porous size, and the synergetic effect between Co3O4 and TiO2, these unique Co3O4@a-TiO2 core–shell microcubes (S-80) exhibit long cycle life, high capacity, and excellent rate capability as advanced electrode materials for LIBs. We anticipate that such a demonstration indicates a versatile method toward preparing other high-performance and multifunctional metal oxides@a-TiO2 nanocomposites for LIBs in near future.Acknowledgements
The authors gratefully acknowledge the financial supports provided by the National Basic Research Program of China (the 973 Project of China, Grant 2011CB935901), National Natural Science Fund of China (Grants 21371108, 21203110), Shandong Provincial Natural Science Foundation for Distinguished Young Scholar (Grant JQ201304), the National Science Foundation of Shandong Province (Grant ZR2012BM018), and the Opening Project of CAS Key Laboratory of Materials for Energy Conversion (Grant KF2014002).References
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J. M. Tarascon, Nature, 2000, 407, 496 CrossRef CAS PubMed.
- J. S. Chen, T. Zhu, Q. H. Hu, J. J. Gao, F. B. Su, S. Z. Qiao and X. W. Lou, ACS Appl. Mater. Interfaces, 2010, 2, 3628 CAS.
- X. W. Lou, D. Deng, J. Y. Lee, J. Feng and L. A. Archer, Adv. Mater., 2008, 20, 258 CrossRef CAS PubMed.
- H. B. Wu, J. S. Chen, H. H. Hng and X. W. Lou, Nanoscale, 2012, 4, 2526 RSC.
- S. L. Xiong, J. S. Chen, X. W. Lou and H. C. Zeng, Adv. Funct. Mater., 2012, 22, 861 CrossRef CAS PubMed.
- J. C. Liu, Y. J. Xu, X. J. Ma, J. K. Feng, Y. T. Qian and S. L. Xiong, Nano Energy, 2014, 7, 52 CrossRef CAS PubMed.
- J. S. Chen, C. M. Li, W. W. Zhou, Q. Y. Yan, L. A. Archer and X. W. Lou, Nanoscale, 2009, 1, 280 RSC.
- W. W. Zhou, C. W. Cheng, J. P. Liu, Y. Y. Tay, J. Jiang, X. T. Jia, J. X. Zhang, H. Gong, H. H. Hng, T. Yu and H. J. Fan, Adv. Funct. Mater., 2011, 21, 2439 CrossRef CAS PubMed.
- Y. S. Luo, J. S. Luo, J. Jiang, W. W. Zhou, H. P. Yang, X. Y. Qi, H. Zhang, H. J. Fan, D. Y. W. Yu, C. M. Li and T. Yu, Energy Environ. Sci., 2012, 5, 6559 CAS.
- L. Yu, Z. Y. Wang, L. Zhang, H. B. Wu and X. W. Lou, J. Mater. Chem. A, 2013, 1, 122 CAS.
- L. M. Qin, Q. Zhu, G. R. Li, F. T. Liu and Q. M. Pan, J. Mater. Chem., 2012, 22, 7544 RSC.
- X. M. Wu, S. C. Zhang, L. L. Wang, Z. J. Du, H. Fang, Y. H. Ling and Z. H. Huang, J. Mater. Chem., 2012, 22, 11151 RSC.
- G. D. Du, Z. P. Guo, P. Zhang, Y. Li, M. B. Chen, D. Wexler and H. K. Liu, J. Mater. Chem., 2010, 20, 5689 RSC.
- X. Gu, L. Chen, Z. C. Ju, H. Y. Xu, J. Yang and Y. T. Qian, Adv. Funct. Mater., 2013, 23, 4049 CrossRef CAS PubMed.
- X. Y. Xue, Z. H. Chen, L. L. Xing, S. Yuan and Y. J. Chen, Chem. Commun., 2011, 47, 5205 RSC.
- D. W. Kim, I. S. Hwang, S. J. Kwon, H. Y. Kang, K. S. Park, Y. J. Choi, K. J. Choi and J. G. Park, Nano Lett., 2007, 7, 3041 CrossRef CAS PubMed.
- L. L. Xing, Y. Y. Zhao, J. Zhao, Y. X. Nie, P. Deng, Q. Wang and X. Y. Xue, J. Alloys Compd., 2014, 586, 28 CrossRef CAS PubMed.
- Q. Wang, C. Y. Zhang, X. B. Xia, L. L. Xing and X. Y. Xue, Mater. Lett., 2013, 112, 162 CrossRef CAS PubMed.
- X. B. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891 CrossRef CAS PubMed.
- D. H. Wang, D. W. Choi, Z. G. Yang, V. V. Viswanathan, Z. M. Nie, C. M. Wang, Y. J. Song, J. G. Zhang and J. Liu, Chem. Mater., 2008, 20, 3435 CrossRef CAS.
- M. Wagemaker, W. J. H. Borghols and F. M. Mulder, J. Am. Chem. Soc., 2007, 129, 4323 CrossRef CAS PubMed.
- S. H. Liu, H. P. Jia, L. Han, J. L. Wang, P. F. Gao, D. D. Xu, J. Yang and S. N. Che, Adv. Mater., 2012, 24, 3201 CrossRef CAS PubMed.
- Z. Y. Guo, X. L. Dong, D. D. Zhou, Y. J. Du, Y. G. Wang and Y. Y. Xia, RSC Adv., 2013, 3, 3352 RSC.
- Y. Xiao, C. W. Hu and M. H. Cao, Chem.–Asian J., 2014, 9, 351 CrossRef CAS PubMed.
- J. Bai, X. G. Li, G. Z. Liu, Y. T. Qian and S. L. Xiong, Adv. Funct. Mater., 2014, 24, 3012 CrossRef CAS PubMed.
- W. Li, J. P. Yang, Z. X. Wu, J. X. Wang, B. Li, S. S. Feng, Y. H. Deng, F. Zhang and D. Y. Zhao, J. Am. Chem. Soc., 2012, 134, 11864 CrossRef CAS PubMed.
- J. F. Li, J. Z. Wang, D. Wexler, D. Q. Shi, J. W. Liang, H. K. Liu, S. L. Xiong and Y. T. Qian, J. Mater. Chem. A, 2013, 1, 15292 CAS.
- J. F. Li, S. L. Xiong, Y. R. Liu, Z. C. Ju and Y. T. Qian, ACS Appl. Mater. Interfaces, 2013, 5, 981 CAS.
- J. F. Marco, J. R. Gancedo, M. Gracia, J. L. Gautier, E. RÍOs and F. J. Berry, J. Solid State Chem., 2000, 153, 74 CrossRef CAS.
- T. Choudhury, S. O. Saied, J. L. Sullivan and A. M. Abbot, J. Phys. D: Appl. Phys., 1989, 22, 1185 CrossRef CAS.
- J. F. Li, S. L. Xiong, Y. R. Liu, Z. C. Ju and Y. T. Qian, Nano Energy, 2013, 2, 1249 CrossRef CAS PubMed.
- Y. E. Roginskaya, O. V. Morozova, E. N. Lubnin, Y. E. Ulitina, G. V. Lopukhova and S. Trasatti, Langmuir, 1997, 13, 4621 CrossRef CAS.
- J. H. Zhong, A. L. Wang, G. R. Li, J. W. Wang, Y. N. Ou and Y. X. Tong, J. Mater. Chem., 2012, 22, 5656 RSC.
- C. V. Schenck, J. G. Dillard and J. W. Murray, J. Colloid Interface Sci., 1983, 95, 398 CrossRef CAS.
- M. Oku and Y. Sato, Appl. Surf. Sci., 1992, 55, 37 CrossRef CAS.
- T. J. Chuang, C. R. Brundle and D. W. Rice, Surf. Sci., 1976, 59, 413 CrossRef CAS.
- M. A. Langell, M. D. Anderson, G. A. Carson, L. Peng and S. Smith, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 4791 CrossRef CAS.
- E. Mccafferty and J. P. Wightman, Surf. Interface Anal., 1998, 26, 549 CrossRef CAS.
- F. F. Wu, X. J. Ma, J. K. Feng, Y. T. Qian and S. L. Xiong, J. Mater. Chem. A, 2014, 2, 11597 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra06711a |
| This journal is © The Royal Society of Chemistry 2015 |



