Graphene dispersion in hydrocarbon medium and its application in lubricant technology
Jyotiranjan Ota*,
S. K. Hait,
M. I. S. Sastry and
S. S. V. Ramakumar
Nanotechnology Dept., Indian Oil Corp Ltd. R&D Centre, Faridabad, India-121007. E-mail: jyotiranjano@indianoil.in; Fax: +91-129-2286221; Tel: +91-129-2294687
First published on 2nd June 2015
Abstract
Graphene was prepared in a hydrocarbon oil medium through a mechano-chemical process. The resulting dispersion is a mixture of multilayer and single layer graphene as revealed by High Resolution Transmission Electron Microscopy (HRTEM) and Selected Area Electron Diffraction (SAED) studies. The dispersion yielded significant increase in the thermal conductivity with respect to the base oil. The inclusion of the graphene dispersion in grease instead of the traditionally used graphite improved the thermal conductivity value considerably, without deteriorating the tribological properties achieved from the pristine graphite system. A fully formulated lubricant inhibited with the said graphene dispersion showed excellent thermal dissipation and reduction in coefficient of friction over the neat lubricant. Spectro-analytical studies of the used test specimen of tribological test rig bear the signature peak of the graphene film, thus proving the lubrication efficiency of graphene in hydrocarbon medium.
1 Introduction
Graphite has been a main constituent of a certain category of lubricating greases for decades as it improves the tribological properties as well as allows better thermal conductivity of the system. However, its poor stability in solvents and nonpolar hydrocarbon oils has always been a challenge.1 The recently discovered and widely studied material graphene is very unique with respect to its properties and applications.2–6 This material has the highest thermal conductivity at room temperature, even more than diamond, and also enhances the convective heat transfer of nanofluids.7–9 Hence, the inclusion of this material into lubricant or grease compositions would render unique thermal conductivity properties as heat removal from the system is the primary function of a lubricant. Very recently, reports have been published on graphene as the thinnest solid lubricant and propounded the mechanism of a superlubric system, as evidenced by atomic force microscopy.10,11 Bermen et al. have reported graphene as a good candidate for steel on steel lubrication.12 In a review article, the same group has surveyed the tribological properties of graphene, in particular, its self-lubricating film capabilities or as an additive for lubricating oils.13 Moreover, extraordinary macroscale wear resistance was also reported on a pin-on-disc type system, where a few layers of graphene completely cease wear.14 Based on graphene, value added greases, engine oils and metal working oils have been reported.15–18 In all these reports, tribological and thermal dissipation properties of graphene have been found to be excellent compared to graphite nanoparticles and even carbon nanotubes (CNT). However, all these works use graphene powders as a solid additive to the base fluid, and this process has a serious issue of suspension stability, whereas commercial fluids need very long storage stability.19In order to overcome the above mentioned drawbacks, the functionalization of the graphene sheets with long alkyl chains or aromatic containing hydrocarbon chains followed by re-dispersion in a non-polar oil medium was tried by a few groups.20,21 Zhang et al. dispersed oleic acid modified graphene sheets in PAO and obtained good improvements in friction and antiwear performances.22 However, the concerns of long term storage stability are not resolved for a full fledged commercial use of this new functional material. In this context, the preparation of stable graphene dispersions in hydrocarbon base fluids would be really helpful. This could enhance the suspension stability owing to better interaction with the matrix in terms of polarity balance, and a homogenized dispersion would facilitate the lowering of the coefficient of friction and wear rate effectively at the macroscale. It is to be noted that in situ graphite exfoliation and graphene dispersion in hydrocarbon oils is still not completely attainable, though a few attempts have been made by researchers.23 On a different note, the mechanical exfoliation of the layered materials, more specifically graphite, is relatively simple and is the best method to prepare graphene without any unwanted functionalization/defects. Here, a combination of compressive and shear forces act on the particles, leading to delamination. However, the acting shear force needs to be sufficient enough to overcome the van der Waals force between the sheets. In this regard, there are few reports where either shear ball milling or three roll milling has been used to either prepare graphite nano-sheets or exfoliate graphite to prepare graphene.24–26 The stirred media bead mill system with its unique build provides more shear than impact, which would be more effective for exfoliation. Peukert et al. reported a scalable preparation of graphene in water in the presence of ionic surfactants using a bead mill system.27
Based on this discussion, the use of stirred media mill for mechanical exfoliation in the presence of a suitable dispersant that wraps the sheets and prevents them from re-agglomerating would be worthwhile to investigate with respect to their utility in a non-polar hydrocarbon medium. A method for producing multilayer graphene (MLG) or single layer graphene (SLG) dispersions at industrial scale in a hydrocarbon oil medium would be very useful for their incorporation in greases, lubricants and related applications. The present paper outlines a process of preparing a stable dispersion of graphene in hydrocarbon medium and its inclusion in grease and lubricating oil for improved thermal conductivity and tribological properties. In particular, the graphene system consists of a mixture of MLG and SLG which is a result of delamination/exfoliation of natural graphite. The experimental results described herein indicate the utility of a stable graphene dispersion in lubricant applications.
2 Experimental
2.1 Materials & ingredients
A poly isobutylene succinic imide (PIBSI) based dispersant received from a commercial source and a commercially procured natural graphite with an initial agglomerated particle size of ∼4 microns were used. A number of different hydrocarbon oils such as linear alkyl benzene (LAB), and API Gr I and II lube base oils (with varying hydrocarbon compositions) with different viscosities suitable for specific applications were used.2.2 Premixing and grinding
The premixing step is very important to ensure the effective interaction between the dispersant and pristine graphite with the carrier fluid, leading to effective size reduction. Initially, the calculated amount of dispersant was mixed well in the oil with the help of an over head stirrer. Graphite powder was added to the system slowly with constant stirring in order to get proper mixing. The resulting dispersion was further processed with a stirred media mill system using different size beads sequentially starting from the larger ones to the smaller ones. The chamber was filled with 70% of grinding media and the speed of the rotor was optimized to get the maximum shear force required for exfoliation.2.3 Characterization
Raman spectra of the dispersion, diluted, drop-casted and dried on a glass slide were collected from a SEKI 750 Raman analyzer using an argon ion laser (514.5 nm line). FESEM analysis of the sample was done on a SUPRA 55 instrument of Carl Zeiss make. TEM and HRTEM analyses were done on a diluted, drop-casted and dried sample on a carbon coated copper grid using a JEOL 2100 electron microscope. The thermal diffusivity of the dispersions as well as the prepared formulations was measured with the help of the laser flash method using a Microflash model LFA 457 of NETZSCH. The samples were taken in an aluminum pan, coated with conductive graphite. A triple layer pulse correction method was used for analysis and optimization of the data. The specific heat of the samples was measured on a Q-2000 from TA instruments. Thermal conductivity was calculated from the diffusivity, specific heat and density values. The anti wear (AW) properties of the grease based samples were examined using a four ball wear tester (Falex friction and wear test machine) by following the ASTM standard D4172 method. The LFW-1 test was done on a FALEX wear and friction test instrument using a block-on-ring system. The flat test block used was a FALEX H-60 and the test was performed using 200 lb load@1125 rpm for 3 h.3 Results and discussion
3.1 Structural characterization
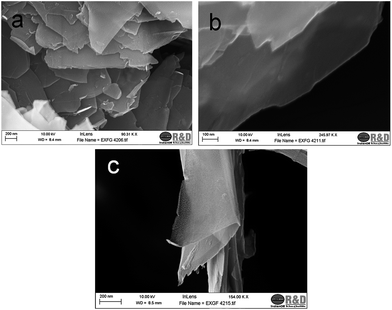
Graphite is a layered material with honey comb like single carbon layers stacked along the c-axis by van der Waals forces. Sequential grinding combined with shearing can lead to the de-stacking of the layers forming exfoliated graphite, which may be identified as MLG, few layered graphene (FLG) or SLG depending upon the number of layers. More de-stacking of the graphite lamellar structure is expected with a low intensity pure shear milling process. Keeping this in mind, the milling process was designed in situ in the hydrocarbon oil medium itself. The obtained material (dispersion) was characterized extensively for morphological and structural confirmation.The morphology study of the final sample was done by FESEM (Fig. 1). This gives the nature of the sample in a micro region and sheet like structures with a wide variation in lateral dimension can be observed (Fig. 1a). It is worth noting that the image is representative of the sample nature and observations more or less the same were found throughout the sample. Fig. 1b shows very thin sheets which are nearly transparent, which may correspond to a few layers of graphene. Another image at high resolution (Fig. 1c) depicts a few curved graphene sheets. These sheets tend to fold in order to minimize the high surface energy.
TEM images revealed that at most of the places thin flat structures of lateral dimension of a few to several hundred nanometers could be found with different thicknesses (based on transparency) as shown in Fig. 2a. In Fig. 2b, a few sheets of nearly 100 nm width and a few hundred nanometer length with a well demarcated edge can be seen. However, at some points the sheets are curled owing to the high surface energy at the edges. They also tend to stack on each other due to the high surface energy after exfoliation. The HRTEM image depicts the lattice fringes having a planar distance of 0.35 nm, suggesting the graphitic nature of the sample. The selected area electron diffraction (SAED) pattern is a good tool to know the number of sheets present in graphene through analyzing the intensity ratio of Bragg reflections. For monolayer graphene, I[1100]/I[2100] > 1.28 In the present SAED pattern collected from the sample, the planes marked in the image have an intensity ratio of ∼1.3, which is a unique feature for monolayer graphene, confirming the presence of monolayer graphene. In addition, the well demarcated hexagonal pattern maintaining the six fold symmetry indicates the good crystallinity of the graphene sheets. In a nutshell, the TEM study suggests the presence of a mixture of well crystalline FLG as well as SLG sheets with cross-sectional dimensions of a few hundred nanometers.
 | ||
| Fig. 2 TEM images of the sample: (a) a sheet on a lacy carbon coated grid, (b) a few sheets at low magnification, (c) HRTEM of graphene, (d) SAED pattern of the sample. | ||
Raman spectroscopy is a powerful tool to distinguish graphite from graphene and to determine the exact number of layers in the latter case. Graphitic materials can be characterized by the D-band around 1350 cm−1, the G-band around 1580 cm−1 and the 2D-band (∼2700 cm−1). The spectrum shown in Fig. 3 depicts the G band at 1582 and 1574 cm−1, and the D band at 1357 and 1351 cm−1 for the graphite and exfoliated graphite samples respectively suggesting the near graphitic nature of the samples. However, for the exfoliated sample the peaks are shifted to the left indicating a more ordered structure and an increase in sp2 carbon density. As documented widely, the symmetry as well as the full width at half maximum (FWHM) of the 2D peak can be employed to distinguish between graphene (monolayer, bilayer or multilayer) and bulk graphite.29 The 2D peak for graphite appears at 2720 cm−1 and the same peak for the exfoliated sample is at 2700 cm−1 indicating delamination and the formation of a few layers or monolayer graphene. Moreover, as it can be seen in the high resolution peak for 2D (inset), the peak appears to be single in nature confirming the sample to be monolayer graphene. We have examined various parts of the diluted film deposited, and could find the single 2D peak in many places. In some places we also found the graphitic nature of the peak. Hence, to conclude from the Raman study, the dispersion contains a mixture of multilayers as well as single layered graphene sheets.
 | ||
| Fig. 3 Raman spectra of the dispersion after grinding. High resolution spectra for the 2D peak (inset). | ||
3.2 Thermal conductivity of the graphene dispersion in base oil
In spite of a fairly good understanding of the theoretical aspects of the thermal conductivity of graphene, only a small amount of experimental data has been reported to date.30–32 This can be explained on the basis of the difficulty of measuring the single layers of graphene by conventional methods such as thermal bridge, the 3ω method or the laser flash method. Hence, most of the reports are either theoretically proposed or rely on Raman optothermal and electrical measurements.33 The laser flash method is a good technique for measuring cross-plane thermal conductivity and requires a substantial temperature drop across the plane and the same has been used for the study of graphene as a thermal interface material.15 The same technique has also been used for obtaining the thermal diffusivity of base oil and different graphene dispersions as well as their blends in formulation. Furthermore, the values have been multiplied by their respective specific heat and density at that temperature to get the thermal conductivity values as per the following equation:| Thermal conductivity (K) = thermal diffusivity (α) × specific heat (Cp) × density (ρ) |
The thermal conductivity values of the neat base oil as well as of that containing 0.5% concentration of the in situ prepared graphene dispersion are shown in Fig. 4. It can be observed that the thermal conductivity of the base oil containing the graphene dispersion is higher at all temperatures studied. The increase in thermal conductivity is in the range of 10–40%, where the composition containing graphene is comparatively more conducting at higher temperature ranges. The improvement is substantial and suggests the graphene dispersion can be used for thermal management in industries. Moreover, the results suggest the graphene dispersion can be used with base oils for high temperature applications.
3.3 Evaluation of the grease containing graphene dispersion
The practical utility of an additive can be best tested in a fully formulated lubricant formulation and the way it behaves in the presence of a number of other additives in the system decides its efficiency. In order to check the performance of the prepared graphene dispersion in comparison to widely used graphite, the thermal conductivity of grease containing the same concentrations of graphene is compared with that of graphite. The grease system consists of a clay-based grease with a required quantity of MoS2 powder, usually a constituent of the above selected grease. In order to make the graphene dispersion compatible, it has been prepared in a highly viscous bright stock (29–32 cSt@100 °C), which is normally the base fluid for clay based high temperature greases. The thermal conductivity values obtained by the abovementioned method (Laser Flash) are shown in Fig. 5. It is evident from the figure that the composition with graphene is a clear winner with a considerably higher thermal conductivity for the whole range of temperatures studied. The grease containing graphene showed an improvement in thermal conductivity of 25–40% in comparison to the composition with the same concentration of graphite. It is worth noting that the thermal conductivity measurement has been done by a laser flash system using a triple layer pulse correction technique. The given geometry in this method allows the heat to propagate from the top to the bottom surface of the material under test. We believe the measured thermal conductivity is more cross plane in nature as already reported by Baladin et al. for thermal interface materials, where they got a significant increase in thermal conductivity.15 They studied the thermal conductivity of a graphene-MLG doped commercial thermal grease sandwiched between two aluminum surfaces and got a thermal conductivity enhancement factor of 1.4. Moreover, a few optothermal measurements have already established a higher thermal conductivity of suspended graphene, where this cross plane thermal conductivity is measured.34,35 Hence, the cross plane thermal conductivity of suspended graphene layers in the grease system enhances thermal conductivity. The higher thermal conductivity would dissipate the heat generated in the system more effectively and help the smooth running of the machinery.Tribological data of the clay based grease containing the graphene dispersion and graphite were generated on a four ball test bench. No differences were found in the weld load for either of these compositions, the values for both being 200 kg under the similar testing conditions. However, the wear scar diameter for the graphene containing composition was found to be significantly lower (∼25%), with a value of 0.70 in comparison to 0.95 for the graphite containing grease. The result clearly indicates a reduction in tribo-corrosion, which results in the lowering of the wear as already reported for sliding steel surfaces by Bermen et al.12 It is believed that the passivation effect due to infringement of graphene sheets into the asperities present on the surface effectively protects the surface, decreasing the wear scar diameter.
3.4 Evaluation of the lubricating oil containing graphene dispersion
Some of the recent studies already reported that a few layers of graphene can be exploited for applications involving rotational and sliding contacts owing to their ease of shear property.11,12 Hence, a combined effect of this shear property and high thermal conductivity can be exploited for lubricants in closed systems. The idea is to add value to existing lubricating oils by top treating with the graphene dispersion. A quantum of the prepared graphene dispersion in bright stock was added to the existing formulation of a commercially available industrial gear oil with the help of mild sonication to homogenize and was subjected to a tribo-analytical test based on stability evaluation.The storage stability of any additive system & compatibility with other additives is very important for a lubricant formulation, as, on a commercial scale, extended shelf life is a critical factor. Even though dispersion is stable enough, its compatibility with the other additives in the final formulation is equally important to get the maximum benefit of the additive. The incompatibility of the additive systems leads to phase separation and sometimes shows an antagonistic behavior leading to the deterioration of the properties. It is worth noting that the graphene dispersions prepared herein are stable at least for one year and do not separate out when incorporated into the complex lubricant formulations. The storage stability has been tested here by putting the graphene dispersion as well as the doped formulations in a measuring cylinder with a stopper and visually observing the formation of an oil layer, if any, at the top and a precipitate at the bottom, if any, by turning it upside down at regular and long intervals. It is also important to note that it does not have any antagonistic effect with the tested formulations.
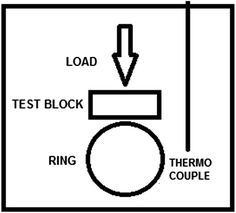
The LFW-1 test is generally used to check the friction and wear characteristics of stable lubricant formulations, and the effective thermal dissipation behavior. A schematic of the setting for the performed test is depicted in Fig. 6. Typically, a static test block is placed over the ring, which is submerged in the test fluid. An operator selected load is applied on the block vertically, while the ring rotates in a reversible manner allowing shear force in contact. A thermocouple is inserted into the closed system to measure the change in temperature inside.
The test results for the neat formulation and that of the top-treated blend with the graphene dispersion have been summarized (Fig. 7). As evident from Fig. 7a, the temperature rise of the system for the top treated formulation has been reduced dramatically. The maximum temperature reached for the neat lubricant was 114 °C and it was 92 °C for the 0.5% top treated formulation. This is a good indication of the effective heat removal capacity of graphene in a closed industrial machine set up. Furthermore, it is to note that for the neat lubricant, the temperature rose to a maximum and remained almost constant for the entire duration of the test run. However, in the case of the lubricant treated with graphene, the temperature started coming down after some time and reached a lower temperature of 74 °C. The friction coefficient as measured from the same test is shown in Fig. 7b, which is 0.07 for the neat gear oil. In the case of the composition treated with graphene, the value is 0.07 initially and it reduces to 0.02 at the end of the test. It seems that the sheets of graphene take some time to infiltrate into the asperities and make a protective film, thereby reducing the coefficient of friction. In order to understand the effective heat dissipation of the graphene dispersion in the LFW-1 test, we have measured the thermal conductivity of the neat as well as the additized gear oil and the results are shown in Fig. 7c. The thermal conductivity of the graphene (0.5%) top treated lubricant is way higher than the neat one. This explains the excellent result of the LFW-1 study and proves graphene is quite effective in dissipating heat. On the other hand, as it can be noted from the temperature study of the LFW-1 test, there is a gradual decrease in the system temperature with elapsing time, suggesting the decrease in heat generated in the system. This could be due to graphene forming a lubricating film and protecting the surface, decreasing friction, and thereby minimizing heat generation. The same is well evidenced by the coefficient of friction values. Hence, a combined effect of lubrication and heat dissipation played a crucial role, which suggests graphene is an excellent multifunctional lubricant additive. It is to be noted that the same industrial gear oil top treated with graphite failed in the LFW-1 test due to a very high rise in the maximum temperature achieved. The maximum temperature rose to nearly 160 °C and the test specimen was corroded heavily with high wear debris. Hence, graphene is established as a better lubricant than graphite and the results are encouraging for the application of graphene in gear oils and other industrial lubricants as a value added additive.
3.5 Analytical studies on the test specimen after the LFW-1 test
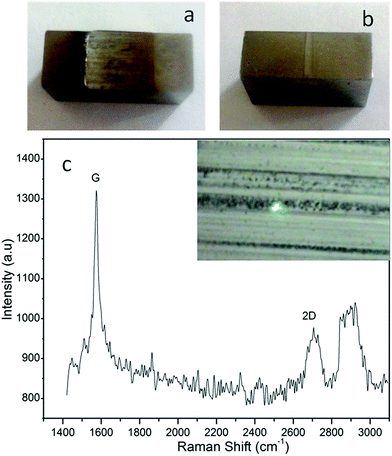
Although graphene has been reported to have better lubricating properties, most of the studies are done on microscopic platforms with the help of scanning probe microscopy. A few reports also exist, in which the wear track after the tribo test was characterized by Raman to find traces of graphene on the wear track.12 Hence, to ascertain the results achieved on the macro test benches, the specimens after the LFW-1 test were characterized further. Before the analysis, the specimens of the LFW-1 test were washed thoroughly with hexane to remove the oil from the surface. Digital photographs of the two representative specimens are shown in Fig. 8. The heavily worn out track for the graphite containing sample (Fig. 8a) suggests the failure of graphite as a lubricant. The track for the graphene containing sample (Fig. 8b) is significantly smoother with less wear. Hence, it is evident that for the graphene containing formulation, less wear is due to the protection by lubricating film formation. In order to know the nature of the film, the wear tracks were observed in the Raman spectroscope fitted with an optical microscope. The 100 times magnified image collected on the microscope along with the Raman spectrum is shown in Fig. 8c. The Raman spectrum was collected from the spot marked by the laser. The spectrum shows the 2D peak is centered around 2700 cm−1 which indicates the presence of a graphene layer on the tribo surface. The peak is also quite singular in nature suggesting a single or bi-layer of graphene. This confirms a permanent tribo-film of graphene formed on the surface, reducing the wear. It can also be noted that the spectrum contains noisy peaks around 2900 cm−1. This could be due to some hydrocarbon footprints of other additives of the fully formulated oil or the surfactant used to stabilize graphene.4 Conclusions
A stable graphene dispersion in a hydrocarbon medium has been prepared by the mechanical exfoliation of natural graphite. The graphene dispersion consists of a mixture of multilayer as well as single layer sheets. The prepared dispersion exhibits a higher thermal conductivity in comparison to the base fluid and the same was manifested in grease and other lubricant formulations. There was a decrease in the wear scar diameter for the tested graphene containing grease in place of graphite. The tribological studies for a lubricating oil system with the graphene dispersion show a dramatic lowering of the rise in maximum temperature and coefficient of friction. It can be concluded that the graphene dispersion has both lubricating properties and higher thermal conductivity. The graphene layer forms a protecting film decreasing friction and wear. The higher thermal conductivity efficiently dissipates the heat, maintaining as well as limiting the temperature rise. These studies hold promise for graphene being a potential additive for various lubricant formulations.References
- S. Niyogi, E. Bekyarova, M. E. Itkis, J. L. McWilliams, M. A. Hamon and R. C. Haddon, J. Am. Chem. Soc., 2006, 128, 7720–7721 CrossRef CAS PubMed.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS PubMed.
- A. K. Geim, Science, 2009, 324, 1530–1534 CrossRef CAS PubMed.
- C. Soldano, A. Mahmood and E. Dujardin, Carbon, 2010, 48, 2127–2150 CrossRef CAS PubMed.
- Y. Zhu, S. Murali, W. Cai, X. Li, J. W. Suk, J. R. Potts and R. S. Ruoff, Adv. Mater., 2010, 22, 3906–3924 CrossRef CAS PubMed.
- X. Huang, Z. Yin, S. Wu, X. Qi, Q. He, Q. Zhang, Q. Yan, F. Boey and H. Zhang, Small, 2011, 7, 1876–1902 CrossRef CAS PubMed.
- K. M. F. Sahil and A. A. Baladin, Solid State Commun., 2012, 152, 1331–1340 CrossRef PubMed.
- T. T. Baby and S. Ramaprabhu, Nanoscale Res. Lett., 2011, 6, 289 CrossRef PubMed.
- W. Yu, H. Xie, X. Wang and X. Wang, Phys. Lett. A, 2011, 375, 1323–1328 CrossRef CAS PubMed.
- K. S. Kim, H. J. Lee, C. Lee, S. K. Lee, H. Jang, J. H. Ahn, J. H. Kim and H. J. Lee, ACS Nano, 2011, 5, 5107–5114 CrossRef CAS PubMed.
- X. Feng, S. Kwon, J. Y. Park and M. Salmeron, ACS Nano, 2013, 7, 1718–1724 CrossRef CAS PubMed.
- D. Bermen, A. Erdemir and A. V. Sumant, Carbon, 2013, 54, 454–459 CrossRef PubMed.
- D. Bermen, A. Erdemir and A. V. Sumant, Mater. Today, 2014, 17, 31–42 CrossRef PubMed.
- D. Bermen, S. A. Deshmukh, K. R. S. Sankaranarayanan, A. Erdemir and A. Sumant, Adv. Funct. Mater., 2014, 24, 6640–6646 CrossRef PubMed.
- K. M. F. Sahil and A. A. Baladin, Nano Lett., 2012, 12, 861–867 CrossRef PubMed.
- X. Fan, Y. Xia, L. Wang and W. Li, Tribol. Lett., 2014, 55, 455–464 CrossRef CAS.
- V. Eswaraiah, V. Sankarnarayanan and S. Ramaprabhu, ACS Appl. Mater. Interfaces, 2011, 3, 4221–4227 CAS.
- J. Samuel, J. Rafiee, P. Dhiman, Z. Z. Yu and N. Koratkar, J. Phys. Chem. C, 2011, 115, 3410–3415 CAS.
- L. R. Rudnik, Lubricant Additives-Chemistry and Applications, Marcel Dekker, New York, 2003 Search PubMed.
- T. Kuila, S. Bose, C. E. Hong, M. E. Uddin, P. Khanra, N. H. Kim and J. H. Lee, Carbon, 2011, 49, 1033–1037 CrossRef CAS PubMed.
- S. Choudhary, H. P. Mungse and O. P. Khatri, J. Mater. Chem., 2012, 22, 21032–21039 RSC.
- W. Zhang, M. Zhou, H. Zhu, Y. Tian, K. Wang, J. Wei, F. Ji, X. Li, Z. Li, P. Zhang and D. Wu, J. Phys. D: Appl. Phys., 2011, 44, 205303 CrossRef.
- A. Zhamu and B. Z. Jang, Nano Graphene Modified Lubricant, U.S patent, US 2011/0046027 A1, 2011.
- M. V. Antisari, A. Montone, N. Jovic, E. Piscopiello, C. Alvani and L. Pilloni, Scr. Mater., 2006, 55, 1047–1050 CrossRef CAS PubMed.
- W. Zhao, M. Fang, F. Wu, H. Wu, L. Wang and G. Chen, J. Mater. Chem., 2010, 20, 5817–5819 RSC.
- J. Chen, M. Duan and G. Chen, J. Mater. Chem., 2012, 22, 19625–19628 RSC.
- C. Knieke, A. Berger, M. Voigt, R. N. K. Taylor, J. Rohrl and W. Peukert, Carbon, 2010, 48, 31963204 CrossRef PubMed.
- J. Coleman, et al., Nat. Nanotechnol., 2008, 3, 563–568 CrossRef PubMed.
- M. S. Dresselhaus, A. Jorio, M. Hofmann, G. Dresselhaus and R. Saito, Nano Lett., 2010, 10, 751–758 CrossRef CAS PubMed.
- J. Ota, S. K. Hait, S. S. V. Ramakumar, B. Basu and R. K. Malhotra, J. Nanosci. Nanotechnol., 2013, 13, 5942–5947 CrossRef CAS PubMed.
- W. Yu, H. Xie and W. Chen, J. Appl. Phys., 2010, 107, 094317 CrossRef PubMed.
- S. S. Gupta, V. M. Siva, S. Krishnan, T. S. Sreeprasad, P. K. Singh, T. Pradeep and S. K. Das, J. Appl. Phys., 2011, 110, 084302 CrossRef PubMed.
- A. Baladin, Nat. Mater., 2011, 10, 569–581 CrossRef PubMed.
- S. Ghosh, I. Calizo, D. Teweldebrhan, E. P. Pokatilov, D. L. Nika, A. A. Balandin, W. Bao, F. Miao and C. N. Lau, Appl. Phys. Lett., 2008, 92, 151911 CrossRef PubMed.
- A. A. Baladin, S. Ghosh, D. L. Nika and E. P. Pakatilov, Fullerenes, Nanotubes, Carbon Nanostruct., 2010, 18, 474–486 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2015 |