Synthesis and characterization of a CuS–WO3 composite photocatalyst for enhanced visible light photocatalytic activity
Abstract
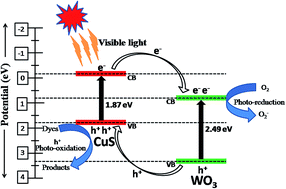
WO3 nanorods and flower-like CuS were synthesized by a hydrothermal process. The visible light driven CuS–WO3 photocatalyst was prepared by adding different weight ratios (10–40%) of CuS on WO3 by a wet impregnation method. The synthesized photocatalysts were characterized by powder X-ray diffraction (XRD), Fourier transform infrared (FT-IR) spectroscopy, Field emission scanning electron microscopy (FE-SEM), High-resolution transmission electron microscopy (HR-TEM), energy dispersive X-ray spectroscopy (EDAX) and UV-vis diffuse reflectance (DRS) spectroscopy. The photocatalytic performance of synthesized photocatalysts was evaluated for the photodegradation of methylene blue (MB) under visible light irradiation. The 10% CuS–WO3 photocatalyst showed higher photocatalytic degradation activity than others which could be due to the increased absorption of light in the visible region and also a lower recombination of charge carriers. Further, the photoelectrochemical measurements carried out for 10% CuS–WO3 revealed the faster migration of photo-induced charge-carriers. A possible reaction mechanism for the enhancement of photocatalytic activity of CuS–WO3 has been proposed.

 Please wait while we load your content...
Please wait while we load your content...