Stainless steel felt as diffusion backing for high-performance microbial fuel cell cathodes
Abstract
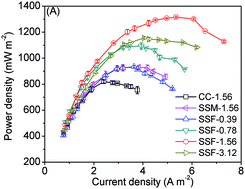
Novel three-dimensional (3D) macroporous cathodes for microbial fuel cells (MFCs) are constructed by using stainless steel felt (SSF) as the diffusion backing and the current collector, instead of two-dimensional (2D) materials such as a carbon cloth (CC) or stainless steel mesh (SSM), thereby resulting in an enlarged surface area for the oxygen reduction reaction (ORR). Different amounts of carbon black (CB) are applied in the base layers to optimize the performance of those SSF cathodes. The MFCs using the SSF cathodes with CB loading of 1.56 mg cm−2 (SSF-1.56) achieve a maximum power density of 1315 ± 6 mW m−2, which is 60% and 42% higher than those using the CC and SSM cathodes, respectively. The results show that the cathode of SSF-1.56 exhibits an excellent catalytic activity for ORR as well as a reduced total internal resistance, thanks to the improved three-phase interface (TPI) that not only facilitates the electron transfer, the proton transfer and the oxygen diffusion, but also offers a large surface for the ORR at the cathode. Our research also demonstrates that the SSF cathodes with an optimal CB loading will benefit the advancement of MFCs in practical application.

 Please wait while we load your content...
Please wait while we load your content...