Controllable synthesis of Ni3−xCoxS4 nanotube arrays with different aspect ratios grown on carbon cloth for high-capacity supercapacitors†
Rui Ding,
Hong Gao*,
MingYi Zhang,
Jing Zhang and
XiTian Zhang*
Key Laboratory for Photonic and Electronic Bandgap Materials, Ministry of Education, School of Physics and Electronic Engineering, Harbin Normal University, Harbin 150025, P. R. China. E-mail: gaohong65cn@126.com; xtzhangzhang@hotmail.com
First published on 25th May 2015
Abstract
Ni3−xCoxS4 (x = 1.5, 2, 2.25, 2.5) nanotube arrays were prepared on carbon cloth by a two-step hydrothermal route and their electrochemical performance was studied. The aspect ratios and electrochemical performance of the Ni–Co sulfide nanotube arrays can be well controlled by manipulating the Co/Ni molar ratio. With the cooperation of the appropriate morphology and compositions, the specific capacitance of Ni0.75Co2.25S4 can reach as high as 1856 F g−1 at 1 A g−1. The rate capability is 88% at a high current density of 50 A g−1. The superior electrochemistry performance demonstrates that Ni–Co sulfide nanotube arrays with an appropriate molar ratio of Co/Ni would be promising for high-performance supercapacitor materials.
1. Introduction
Supercapacitors, a promising energy storage device, also known as electrochemical capacitors, have received tremendous attention in recent years because of their high power densities and excellent cycle life.1–4 To improve the performance of supercapacitors, the electrode material is one of the vital factors. Until now, there are mainly three kinds of electrode materials: carbonaceous materials, transition metal oxides and conducting polymer materials. Among them, transitional metal oxides, such as nickel–cobalt oxides,5,6 have been widely explored for high-performance supercapacitors because of their high theoretical specific capacitance (SC).5 However, their conductivity is typically too low to support fast electron transport toward high practical capacitance and high rate capability. To overcome this problem, recently, nickel–cobalt sulfides, with about 100 times as high as electrical conductivity of NiCo2O4,7–9 have been tried as a new type of electrode materials and good electrochemical performance has been achieved. Thus, Ni–Co sulfides could be more interesting electrode materials compared to Ni–Co oxides. For example, Li and coworkers10 synthesized Ni–Co oxides and Ni–Co sulfides (Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co = 1
Co = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) which delivered a SC of 1222 F g−1 and 2053 F g−1 at a current density of 5 mA cm−2, respectively. Peng et al.11 reported the SC was about 324, 1575 F g−1 for NiCo2O4 and NiCo2S4 at a current density of 10 mA cm−2, respectively. Liu et al.12 produced 3D porous NiCo2S4 whose SC was even 2.5 times higher than that of the NiCo2O4 nanosheets at a current density of 1 A g−1. Furthermore, the Ni–Co sulfides offer richer redox reactions than the corresponding single component sulfides in much the same way as NiCo2O4 to the corresponding single component oxides.13,14 Therefore, Ni–Co sulfides upgraded from single component sulfides and Ni–Co oxides could be the promising electrode materials for supercapacitors.
1) which delivered a SC of 1222 F g−1 and 2053 F g−1 at a current density of 5 mA cm−2, respectively. Peng et al.11 reported the SC was about 324, 1575 F g−1 for NiCo2O4 and NiCo2S4 at a current density of 10 mA cm−2, respectively. Liu et al.12 produced 3D porous NiCo2S4 whose SC was even 2.5 times higher than that of the NiCo2O4 nanosheets at a current density of 1 A g−1. Furthermore, the Ni–Co sulfides offer richer redox reactions than the corresponding single component sulfides in much the same way as NiCo2O4 to the corresponding single component oxides.13,14 Therefore, Ni–Co sulfides upgraded from single component sulfides and Ni–Co oxides could be the promising electrode materials for supercapacitors.
Apart from intrinsic property of active materials, it is well known that the morphologies, structures and sizes of the materials have significant influence on the performance of electrode materials. Different morphologies of Ni–Co sulfides, such as urchin-, tube-, flower-, and cubic-like structures, have been investigated.15 Among those different nanostructures, nanotubes with large specific surface area will provide more electroactive sites contacting with electrolyte ions for faradic energy storage. Then, Ni–Co sulfides with tubular structure were reported recently.16,17
Molar ratio of Co/Ni in Ni–Co sulfides is another important factor for the electrochemical performance of Ni–Co sulfides. Very recently, Chen et al.18 synthesized porous Ni3−xCoxS4 (x = 0, x = 1, x = 1.5, x = 2, x = 3) nanoparticles with different Co/Ni molar ratios and discussed the influence of Co/Ni molar ratio on the electrochemical performance of Ni–Co sulfides. As a result, the Ni1.5Co1.5S4 sample showed the highest SC of 1093 F g−1 at 1 A g−1. Another report19 about Ni3−xCoxS4 hollow nanoprisms mainly focused on the formation of the beautiful hollow nanoprisms and discussed electrochemical performance of NiCo2S4 and Ni2CoS4. Ni2CoS4 delivered a high SC of 895.2 F g−1 at 1 A g−1. Nevertheless, there are few reports about effect of the compositions of Ni–Co sulfides on their electrochemical performance.
Herein, Ni3−xCoxS4 nanotube arrays were designed and synthesized on carbon cloth (CC) with different Co/Ni molar ratios by changing the molar ratio of Ni(NO3)2·6H2O and Co(NO3)2·6H2O in synthesis process. The effect of the compositions of Ni3−xCoxS4 nanotube arrays on their electrochemical performance was studied, and Ni0.75Co2.25S4/CC exhibits a high electrochemical performance.
2. Experimental
2.1. Materials
Commercial CC is used as the current collector. Ni(NO3)2·6H2O, Co(NO3)2·6H2O, urea, thioacetamide (TAA), and other reagents in this experiment are of analytical purity and used without further purification.2.2. Synthesis of Ni–Co precursors
Ni–Co precursors were synthesized by a typical hydrothermal route. CC (2.4 × 3 cm2) was rinsed in acetone, absolute ethanol and sulfuric acid in an ultrasound bath for 30 min, respectively, and then washed with deionized (DI) water. Urea (0.15 g) and a mixture (0.75 g) of Ni(NO3)2·6H2O and Co(NO3)2·6H2O (the molar ratios of Co/Ni are 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 3
1 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 5
1 and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively) were respectively dissolved into 20 mL of DI water and stirred at room temperature, then formed four clear pink solution. The solution was transferred into the 25 mL Teflon-lined stainless steel autoclave. A piece of CC vertically inserted into the Teflon-lined stainless steel autoclave was soaked in the solution and kept at 140 °C for 14 h to form Ni–Co precursors on the CC. The as-synthesized precursors were washed by DI water.
1, respectively) were respectively dissolved into 20 mL of DI water and stirred at room temperature, then formed four clear pink solution. The solution was transferred into the 25 mL Teflon-lined stainless steel autoclave. A piece of CC vertically inserted into the Teflon-lined stainless steel autoclave was soaked in the solution and kept at 140 °C for 14 h to form Ni–Co precursors on the CC. The as-synthesized precursors were washed by DI water.
2.3. Synthesis of Ni3−xCoxS4 nanotubes
Following a typical procedure, 0.2 g TAA was dissolved into 40 mL DI water under magnetic stirring, and then transferred into a 50 mL Teflon-lined stainless steel autoclave. The CC with Ni–Co precursors was vertically inserted into the Teflon-lined stainless steel autoclave and maintained at 160 °C for 14 h. After washed with DI water and followed by vacuum-drying under 60 °C for 14 h, the corresponding Ni3−xCoxS4 nanotube arrays as active materials were obtained based on Kirkendall effect.20 The active materials of Ni3−xCoxS4 with different molar ratios of Co/Ni are denoted as Co-x (x = 1.5, 2, 2.25 and 2.5) in order to be described easily. The reactions could be described by the following equations (eqn (1)–(3)):| CH3CSNH2 + H2O → CH3(NH2)C(OH)–SH | (1) |
| CH3(NH2)C(OH)–SH + H2O → CH3(NH2)C(OH)2 + H2S | (2) |
| H2S + (Ni3−xCox)(Co3)1.5(OH)3 →Ni3−xCoxS4 + H2O + CO2 | (3) |
2.4. Characterization and electrochemical measurements
The morphologies and crystal structures of the samples were directly examined by scanning electron microscopy (SEM, Hitachi SU 70) equipped with an energy-dispersive X-ray spectroscopy detector (EDX), transmission electron microscopy (TEM; FEI, Tecnai TF 20) equipped with selected area electron diffraction (SAED), and X-ray diffraction (XRD; Dlmax 2600, Rigaku, Japan) using Cu Kα radiation (λ = 1.5418 Å), respectively. The electrochemical characterization was carried out in a standard three electrode system by an electrochemical workstation (VMP3, France), where the electroactive materials grown on the CC (1 cm × 1 cm) as the working electrode, Pt foil as the counter electrode, Hg/HgO as the reference electrode, and 3.0 M KOH aqueous solution as the electrolyte. All potentials were referred to the reference electrode. Cyclic voltammetry (CV) was carried out between −0.1 and 0.75 V at scan rates varying from 2 to 50 mV s−1. Galvanostatic charge–discharge (GCD) behavior was evaluated between 0 and 0.5 V at the current densities of 1, 2, 4, 6, 10, 20, 30 and 50 A g−1, respectively. Electrochemical impedance spectroscopy (EIS) tests of the electrode were performed in the frequency range 100 kHz to 0.01 Hz.3. Results and discussion
3.1. Morphology and structure
In order to investigate evolution of the morphology with the change of the Co/Ni molar ratio, single carbon fiber with active materials and corresponding cross-sectional SEM images of the Co-1.5/CC, Co-2/CC, Co-2.25/CC and Co-2.5/CC are shown in Fig. 1. The Ni–Co sulfide nanoarrays vertically align on carbon fiber uniformly (Fig. 1a, d, g and j). It is noteworthy that the length of the prepared samples is depended strongly on the Co/Ni molar ratio. With the increase of Co/Ni molar ratio, Ni–Co sulfide arrays become longer and longer while the diameter remains unchanged as ∼120 nm, as shown in Fig. 1b, e, h and k. The length of arrays for Co-1.5, Co-2, Co-2.25 and Co-2.5 is 1–2 μm, 3–4 μm, 6–8 μm and about 10 μm, respectively. It can be seen from Fig. 1k that the arrays of Co-2.5 collapse and the ends of them touch each other due to the large aspect ratio. The change of the aspect ratio for the nanoarrays may be attributed to the different growth habits of Ni(OH)2 and Co(OH)2 crystal nanostructures.6 The similar phenomenon was observed in Ni3−xCoxS4 hollow nanoprisms.19 From the cross-sectional SEM images in Fig. 1c, f, i and l, there is a thin film of Ni–Co sulfides between the CC and the corresponding Ni–Co sulfide nanotube arrays. With the increase of aspect ratio, the Ni–Co sulfide film becomes thinner and thinner. The thickness of the film of Co-1.5, Co-2, Co-2.25 is about 1.2, 0.6 and 0.2 μm, respectively. As for Co-2.5, the film is so thin that nanotube arrays are nearly grown on CC directly. The different morphologies for the four samples could result in different electrochemical performance. The molar ratio of Co/Ni in the Ni–Co sulfides is consistent with that of Ni(NO3)2·6H2O and Co(NO3)2·6H2O used in synthesis from the EDX data (Fig. S1 and Table S1†). | ||
| Fig. 1 SEM images of the Co-x nanotube arrays grown on single carbon fiber, corresponding cross-sectional SEM images of Ni–Co sulfides, (a–c) Co-1.5, (d–f) Co-2, (g–i) Co-2.25, (j–l) Co-2.5. | ||
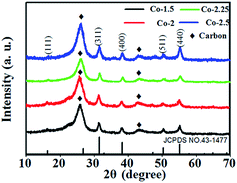
The as-prepared Co-1.5/CC, Co-2/CC, Co-2.25/CC and Co-2.5/CC samples were characterized via XRD. For Co-1.5, Co-2, Co-2.25 and Co-2.5, all the diffraction peaks can be indexed to the NiCo2S4 (JCPDS 43-1477) (shown in Fig. 2). Attributed to the adjacent location of Ni with Co in the periodic table and the similar radius of the Ni and the Co atom, all the peaks for Ni–Co sulfides hardly shift with the increase of the Co/Ni molar ratio. The result is the same as previous works.18,19 EDX analysis of the samples reveals that the as-obtained samples synthesized on CC are Co-1.5, Co-2, Co-2.25 and Co-2.5, respectively.
The detailed microstructures of the as-synthesized Ni–Co sulfides were further provided by TEM, as shown in Fig. 3. Apparently, the four synthesized Ni–Co sulfide arrays are hollow internally based on Kirkendall effect, which is widely employed to create hollow nanostructures for metal sulfides.20 Kirkendall effect is basically attributed to the relative diffusion process through an interface of sulfide ions and metal ions. Because the outward diffusion of metal ions became dominant compared with the inward diffusion of sulfide ions, nanotube wall of Ni–Co sulfides and the hollow interior were finally formed. Those tubular structures have similar diameter about 120 nm and similar wall thickness about 30 nm.
Fig. 4 is composed of four corresponding 1/4 SAED patterns of the (a) Co-1.5, (b) Co-2, (c) Co-2.25 and (d) Co-2.5. The SAED patterns demonstrate the polycrystalline characteristics of the samples by the well-defined diffraction rings. The diffraction rings in Fig. 4a–d can be fully indexed to the (111), (220), (311), (400), (511) and (440) crystal planes of the cubic NiCo2S4 (JCPDS 43-1477) phase. Obviously, the four samples show much the same SAED pattern, which is consistent with the XRD results, further demonstrating their highly similarity in crystal structure.
3.2. Electrochemical properties of the Co-x/CC
The electrochemical performance of the four Co-x/CC samples was evaluated by CV and GCD measurements in a three electrode configuration with an Hg/HgO reference electrode in 3 M KOH aqueous electrolyte. Fig. 5a shows typical CV curves at scan rate of 20 mV s−1 in a potential window from −0.1 to 0.75 V for the four electrodes. Apparently, a cathodic peak and two anodic peaks can be observed in the CV curves for Co-2/CC, Co-2.25/CC, Co-2.5/CC, and one pair redox peak is found for Co-1.5/CC sample, which indicates the rich redox reactions of the Ni–Co sulfides in the electrochemical process.21 The broad redox reaction peaks of the Ni–Co sulfides are from the reversible redox processes of Co2+/Co3+/Co4+ and Ni2+/Ni3+ in KOH.17,22 The reasonable reactions are proposed for the electrochemical reactions (eqn (4)–(6)).23| NiS + OH− ↔ NiSOH + e− | (4) |
| CoS + OH− ↔ CoSOH + e− | (5) |
| CoSOH + OH− ↔ CoSO + H2O + e− | (6) |
The anodic peaks (indicated by p1 in Fig. 5a) of the four Co-x/CC in the CV curves include the redox reaction of Ni2+/Ni3+ and Co3+/Co4+,17,22 Obviously, with the increase of the Co/Ni molar ratio, the peak current of the p1 of CV curves increases from Co-1.5 to Co-2.25 then decreases to Co-2.5, and the position of the anodic peak (p1) is shifted to negative potential direction which can be ascribed to the relatively lower potential of redox reaction between Co3+ and Co4+ compared with Ni2+/Ni3+.18 While there is the other anodic peaks (indicated by p2 in Fig. 5a) coming from the redox reaction of Co2+/Co3+.17,22 With the increase of the Co/Ni, the current of p2 becomes higher and higher relative to p1. No apparent p2 is found for the Co-1.5 because the Co/Ni molar ratio is too low for Co-1.5 and the electrochemical activity of Co2+/Co3+ is too weak compared with Co3+/Co4+ or Ni2+/Ni3+. The reason why only one cathodic peak can be observed apparently in Fig. 5a needs further investigation. Combined the action of p1 and p2, the enclosed area of Co-2.25 is the largest among the four samples, indicating its highest SC. The detailed analysis will be shown later.
Typical CV curves of Co-2.25/CC electrode shown in Fig. 5b are recorded with various scan rates at 5, 10, 20 and 40 mV s−1 in a potential range of −0.1 to 0.75 V and those of Co-1.5/CC, Co-2/CC, and Co-2.5/CC were shown in Fig. S2a–c,† respectively. And with the increase of scan rate from 5 to 40 mV s−1, the positions of anodic and cathodic peaks shift to a more anodic and cathodic direction for the four samples, respectively. It is mainly due to the internal resistance of the electrode.
Fig. 5c shows the GCD curves of the four Co-x/CC samples in the range of 0 to 0.5 V at a current density of 1 A g−1. Two pairs of distinct plateau regions (plateau region 1 and plateau region 2) in the charge and discharge curves for Co-x/CC (x = 2, 2.25, 2.5) and one for Co-1.5/CC match well with the redox reaction peaks observed in the CV curves. At a given current density of 1 A g−1, the SC of Co-1.5/CC, Co-2/CC, Co-2.25/CC and Co-2.5/CC are 1153 F g−1, 1504 F g−1, 1856 F g−1 and 1119 F g−1, respectively, based on the eqn (7).
 | (7) |
| Sample | Specific capacitance | Rate retention | Reference |
|---|---|---|---|
| NiCo2S4 urchin-like | 1149 F g−1 at 1 A g−1 | 66.2% (from 1 to 50 A g−1) | 7 |
| 3D cauliflower-like NiCo2S4 | 1471 F g−1 at 1 A g−1 | 63.9% (from 1 to 50 A g−1) | 9 |
| Ni–Co sulfide nanowires | 2415 F g−1 at 2.5 mA cm−2 | 48.7% (from 2.5 to 30 mA cm−2) | 10 |
| NiCo2S4 nanotubes | 933 F g−1 at 1 A g−1 | 59% (from 1 to 5 A g−1) | 17 |
| Porous Ni1.5Co1.5S4 | 1093 F g−1 at 1 A g−1 | 69% (from 1 to 50 A g−1) | 18 |
| NiCo2S4 hollow nanoprisms | 895.2 F g−1 at 1 A g−1 | 65.4% (from 1 to 20 A g−1) | 19 |
| NiCo2S4 nanotube | 738 F g−1 at 4 A g−1 | 78% (from 4 to 32 A g−1) | 23 |
| Ni0.75Co2.25S4 nanotube | 1856 F g−1 at 1 A g−1 | 88% (from 1 to 50 A g−1) | Present work |
The corresponding SC for different Co-x/CC versus current density is shown in Fig. 6a. The SC remains about 20%, 64%, 88% and 71% for Co-1.5/CC, Co-2/CC, Co-2.25/CC and Co-2.5/CC, respectively, as the current density increases from 1 A g−1 to 50 A g−1. Especially, the high rate capability of Co-2.25/CC electrode which is higher than Ni–Co oxide6,24 and Ni–Co sulfide electrodes obtained before (Table 1) can be ascribed to the improved electrical conductivity and rich electroactive sites of Co-2.25.
To evaluate the electrical resistance responses of the Co-x/CC, EIS tests were carried out at open circuit potential in the frequency range from 100 kHz to 0.01 Hz with an ac perturbation of 10 mV. Fig. 6b shows the Nyquist plots of the four Co-x/CC samples and their enlarged view at high frequency region (inset). The curves are composed of three parts. The intersection on the real axis represents the internal resistance of the electrochemical system (Rs) which includes resistance of the electrode, electrolyte and contact resistance at the interface between electrolyte and electrode. The magnitudes of Rs obtained from the four samples are quite low and the Rs of Co-2.25/CC sample is the lowest (about 0.71 Ω). The diameter of the semicircle at a high frequency region is corresponding to the charge-transfer resistance (Rct) at the electrode-electrolyte interface. With the increase of Co/Ni molar ratio, it should be noted that the diameter of the semicircle for the four samples decreases and then increases. The smallest diameter of the Co-2.25/CC means the easiest charge transport. The results of Rct for the four Co-x/CC samples with different Co/Ni ratios explain well the rate capability of them. Low charge-transfer resistance avoids time constraint and enhances utilization of active surface of electrode materials at high-rate charge–discharge process. The linear part at low frequency represents typical capacitor behavior. The more vertical the line is, the more closely the supercapacitor behaves as an ideal capacitor. The EIS spectra are fitted based on the equivalent circuit model in the inset of Fig. 6b. Besides Rs and Rct, Cdl and Wo represent the double-layer capacitance and Warburg resistance, respectively. Among all, the Co-2.25/CC exhibits a highest slope which means the most superior capacitive performance. Therefore, the remarkable capacitive characteristic, together with its low charge-transfer resistance of Co-2.25/CC demonstrates that the Co-2.25/CC electrode is favorable for excellent electrochemistry performance which is in good agreement with the CV, GCD and rate stability results discussed above.
Cycle stability is another key parameter in relation to the performance of supercapacitors. Cycle stability of Co-2.25/CC was evaluated by the repeated charging–discharging measurement at a constant current density of 10 A g−1 in the potential range of 0 to 0.5 V for 3000 cycles. As shown in Fig. 7, Co-2.25/CC possesses high electrochemical stability and 75.3% of the initial SC is still retained after 3000 cycles. Shapes of the last five cycles remain almost the same to the first five cycles (insets in Fig. 7), which illustrates the excellent long-term cyclic performance of Co-2.25/CC electrode.
Why the Co-2.25/CC shows the best SC among the four prepared Co-x/CC? This can be attributed to the ideal morphology and the appropriate compositions of the active material. The theoretical capacitance of pseudocapacitive electrode materials can be calculated according to the formula:25
| SC = (n × F)/(M × V) | (8) |
Clearly, the Co-2.25/CC seems to be the most promising one among the four prepared Co-x/CC. The excellent electrochemical performance of Co-2.25/CC can be attributed to the following factors. First, the high specific surface area deriving from the tube-nanostructure will possess many electroactive sites, which can significantly facilitate redox reactions. Second, the cooperation of the appropriate aspect ratio and compositions of the active material enhances the capacitive properties of the active materials. Third, the direct growth of Ni–Co sulfide nanotube arrays on CC which serves as the current collector can ensure good electron conductivity.
4. Conclusions
In conclusion, a series of Ni–Co sulfide nanotube arrays with different Ni and Co content have been prepared successfully on CC by a two-step hydrothermal route using Ni–Co precursor nanorods as hard templates. The morphology and electrochemical performance of the Ni–Co sulfides can be controlled by manipulating the Co/Ni molar ratio. It is found that Ni0.75Co2.25S4/CC electrode can display excellent SC of 1856 F g−1 at 1 A g−1, 88% retention of its initial values with increasing the current density from 1 to 50 A g−1. The excellent electrochemical performance of Ni0.75Co2.25S4/CC can be attributed to appropriate compositions of active materials. These findings of the Ni–Co sulfide nanotube arrays suggest a promising electrode material with tunable capacitive behavior and may open up great opportunities for applications in supercapacitors.Acknowledgements
This work was partially supported by the Natural Science Foundation of China (no. 51172058, 51472066 and 51402076), the Natural Science Foundation of Heilongjiang Province (ZD201112 and QC2014C056) and Institution of Higher Education Doctoral Fund Jointly Funded Project (20112329110001).References
- Y. W. Zhu, S. Murali, M. D. Stoller, K. J. Ganesh, W. W. Cai, P. J. Ferreira, A. Pirkle, R. M. Wallace, K. A. Cychosz, M. Thommes, D. Su, E. A. Stach and R. S. Ruoff, Science, 2011, 332, 1537–1541 CrossRef CAS PubMed.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- M. Ghidiu, M. R. Lukatskaya, M. Q. Zhao, Y. Gogotsi and M. W. Barsoum, Nature, 2014, 516, 78–81 CAS.
- L. F. Shen, L. Yu, H. B. Wu, X. Y. Yu, X. G. Zhang and X. W. Lou, Nat. Commun., 2015, 6, 6694–6701 CrossRef CAS PubMed.
- L. Huang, D. C. Chen, Y. Ding, S. Feng, Z. L. Wang and M. L. Liu, Nano Lett., 2013, 13, 3135–3139 CrossRef CAS PubMed.
- Y. M. Wang, X. Zhang, C. Y. Guo, Y. Q. Zhao, C. L. Xu and H. L. Li, J. Mater. Chem. A, 2013, 1, 13290–13300 CAS.
- H. C. Chen, J. J. Jiang, L. Zhang, H. Z. Wan, T. Qi and D. D. Xia, Nanoscale, 2013, 5, 8879–8883 RSC.
- J. W. Xiao, X. W. Zeng, W. Chen, F. Xiao and S. Wang, Chem. Commun., 2013, 49, 11734–11736 RSC.
- Y. L. Xiao, Y. Lei, B. Z. Zheng, L. Gu, Y. Y. Wang and D. Xiao, RSC Adv., 2015, 5, 21604–21613 RSC.
- Y. H. Li, L. J. Cao, L. Qiao, M. Zhou, Y. Yang, P. Xiao and Y. H. Zhang, J. Mater. Chem. A, 2014, 2, 6540–6548 CAS.
- T. Peng, Z. Y. Qian, J. Wang, D. L. Song, J. Y. Liu, Q. Liu and P. Wang, J. Mater. Chem. A, 2014, 2, 19376–19382 CAS.
- Y. Liu, J. N. Zhang, S. P. Wang, K. X. Wang, Z. M. Chen and Q. Xu, New J. Chem., 2014, 38, 4045–4048 RSC.
- J. F. Li, S. L. Xiong, Y. R. Liu, Z. C. Ju and Y. T. Qian, ACS Appl. Mater. Interfaces, 2013, 5, 981–988 CAS.
- S. J. Peng, L. L. Li, C. C. Li, H. T. Tan, R. Cai, H. Yu, S. Mhaisalkar, M. Srinivasan, S. Ramakrishna and Q. Y. Yan, Chem. Commun., 2013, 49, 10178–10180 RSC.
- Y. F. Zhang, M. Z. Ma, J. Yang, C. C. Sun, H. Q. Su, W. Huang and X. C. Dong, Nanoscale, 2014, 6, 9824–9830 RSC.
- J. W. Xiao, L. Wan, S. H. Yang, F. Xiao and S. Wang, Nano Lett., 2014, 14, 831–838 CrossRef CAS PubMed.
- H. Z. Wan, J. J. Jiang, J. W. Yu, K. Xu, L. Miao, L. Zhang, H. C. Chen and Y. J. Ruan, CrystEngComm, 2013, 15, 7649–7651 RSC.
- H. C. Chen, J. J. Jiang, Y. D. Zhao, L. Zhang, D. Q. Guo and D. D. Xia, J. Mater. Chem. A, 2014, 3, 428–437 Search PubMed.
- L. Yu, L. Zhang, H. B. Wu and X. W. Lou, Angew. Chem., 2014, 126, 3785–3788 CrossRef PubMed.
- L. Hu, N. Yan, Q. W. Chen, P. Zhang, H. Zhong, X. R. Zheng, Y. Li and X. Y. Hu, Chem.–Eur. J., 2012, 18, 8971–8977 CrossRef CAS PubMed.
- L. Zhang, H. B. Wu and X. W. Lou, Chem. Commun., 2012, 48, 6912–6914 RSC.
- L. Mei, T. Yang, C. Xu, M. Zhang, L. B. Chen, Q. H. Li and T. H. Wang, Nano Energy, 2014, 3, 36–45 CrossRef CAS PubMed.
- J. Pu, T. T. Wang, H. Y. Wang, Y. Tong, C. C. Lu, W. Kong and Z. H. Wang, ChemPlusChem, 2014, 79, 577–583 CrossRef CAS PubMed.
- G. Q. Zhang and X. W. Lou, Sci. Rep., 2013, 3, 1470–1476 Search PubMed.
- C. Zhou, Y. W. Zhang, Y. Y. Li and J. P. Liu, Nano Lett., 2013, 13, 2078–2085 CrossRef CAS PubMed.
- Q. Yang, X. T. Zhang, M. Y. Zhang, Y. Gao, H. Gao, X. C. Liu, H. Liu, K. W. Wong and W. M. Lau, J. Power Sources, 2014, 272, 654–660 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra06440f |
| This journal is © The Royal Society of Chemistry 2015 |