Fluorine-free superhydrophobic/hydrophobic polybenzoxazine/TiO2 films with excellent thermal stability and reversible wettability†
Wenfei Zhang,
Xin Lu,
Zhong Xin*,
Changlu Zhou and
Juan Liu
Shanghai Key Laboratory of Multiphase Structural Materials Chemical Engineering, State Key Laboratory of Chemical Engineering, School of Chemical Engineering, East China University of Science and Technology, 130 Meilong Road, Xuhui District, Shanghai 200237, People’s Republic of China. E-mail: xzh@ecust.edu.cn; Fax: +86-21-64251772; Tel: +86-21-64240862
First published on 10th June 2015
Abstract
Two important properties are combined: the low surface free energy property of polybenzoxazine (PBZ) and the photo-induced superhydrophilicity property of TiO2. A benzoxazine monomer, synthesized by a one-step method, is incorporated with TiO2. Polybenzoxazine/titanium dioxide (PBZ/TiO2) nanocomposite films are prepared using spin coating and a thermal curing method. As a result, a superhydrophobic PBZ/TiO2 film with the largest water contact angle (CA) of ∼166 ± 1° is developed by incorporating a low content of TiO2 (11 wt%, relative to the weight of the benzoxazine monomer) into a PBZ system without any fluorine-containing surface modification agents. The as-prepared superhydrophobic film has good adhesion to glass substrates, and its superhydrophobicity is stable even after heat treatment at 300 °C for 1 h and environmentally durable for more than half a year. When the content of TiO2 is increased to 60%, the consequent nanocomposite film exhibits hydrophobicity–superhydrophilicity transitions with a variation of around 125° in water CA upon ultraviolet (UV) exposure–heat treatment cycles. The effects of the content of TiO2 on the surface wettability, morphology and reversibly switchable wettability of the PBZ/TiO2 films are investigated in detail.
Introduction
Bioinspired superhydrophobic surfaces which possess the merit of a water contact angle (CA) larger than 150° have numerous applications such as in self-cleaning,1,2 oil/water separation,3 antifogging,4 drag reduction,5 microfluidics6,7 as well as in liquid transportation.8 To develop smart surfaces with controllable and specific wettability for use in microfluidic devices or drug containers, a great deal of work has been devoted to constructing reversibly switchable surfaces that are commonly induced by external stimuli such as ultraviolet (UV) light,9,10 heat11 and pH.12As is well known, chemical modification of a rough surface is beneficial to its superhydrophobicity. Conventionally, the modification agent is a fluorine-containing low surface free energy material such as perfluorocarbon or fluoroalkylsilane,13 and the roughening of a surface involves sophisticated processes such as etching,14 templating15 and nanoimprint lithography.16 Therefore, it is desirable to prepare superhydrophobic films by a cost-efficient and an easier process. As a new type of low surface free energy material, polybenzoxazine (PBZ) is promising for superhydrophobic films due to its cross-linking structure, low water absorption, high thermal-stability and low cost.17–19 Chang et al. have fabricated a superhydrophobic film from an equivalent content of PBZ and SiO2 by a two-step casting method, in which a large amount of silica has a detrimental influence on the adhesion performance between the film and a substrate.20,21 Zhang et al. have prepared a superhydrophobic film with a water CA of 152° from the same amount of PBZ and CNTs.22 A fluorinated PBZ/SiO2 film could keep its superhydrophobicity below 250 °C.23,24 In particular, a reversibly switchable PBZ composite surface has been developed from a vinyl-terminated benzoxazine (0.5 g) and SiO2 (0.75 g) through a two-step casting method as well,25 in which the vinyl-terminated benzoxazine was proven to be degradable by UV light while SiO2 was non-photosensitive. Generally, it is preferred to have a relatively small amount of inorganic particles in these composite-prepared films by using a simple preparation method, thus maintaining good adhesion of the prepared film to the substrate and the durability of its superhydrophobic surface property.
Furthermore, extensive interest has been aroused in photo-induced switchable surface wettability, since these surfaces are promising for use in microfluidic devices or drug containers, because of the ability to exhibit efficient remote control under light stimuli.10,26,27 To develop functionalized surfaces with switchable wettability induced by UV light, photo-induced superhydrophilic titanium dioxide nanoparticles (TiO2 NPs) have been widely utilized because of their low cost and non-toxic property, while rough TiO2 surfaces have been generally modified with fluorine-containing materials to achieve superhydrophobicity.9 Considering the practicability and economy of a smart surface, further investigations are required to develop a simple and an effective approach to realize functionalized surfaces with switchable wettability, as well as good adhesion, stability and durability.
According to our preliminary work, a fluorine-free silane-functionalized PBZ film with a surface free energy of 14.91 mJ m−2 has been synthesized.19 Herein, we prepare a superhydrophobic film by spin coating the nanocomposite of silane-functionalized PBZ and TiO2 NPs without any fluorine-containing surface modification agent. The film exhibits strong adhesion to glass substrates and its superhydrophobicity is stable at high temperature. A polybenzoxazine/titanium dioxide (PBZ/TiO2) film with reversible wettability between high hydrophobicity and superhydrophilicity is also achieved.
Results and discussion
Wettability and surface morphology of PBZ/TiO2 film
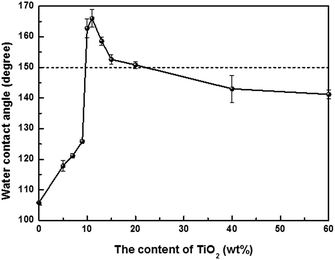
The pure PBZ film has a water CA of ∼106 ± 1° and a superhydrophobic film can be achieved directly by incorporating TiO2 NPs into a benzoxazine monomer after further curing. Variations in the water CAs of PBZ/TiO2 films with different content of TiO2 are shown in Fig. 1 and S2.† The superhydrophobic film with the largest static water CA of ∼166 ± 1° can be prepared when the content of TiO2 is increased to 11%, which is only one tenth of that of SiO2 in the reported PBZ/SiO2.20 Subsequently, there is an observation that the CA experiences a slight and continuous reduction when the content of TiO2 is further increased, which can be explained by the roughness and the surface morphology of each film.The representative roughnesses, water CAs, water contact angle hysteresis (CAH) and sliding angles (SAs) of PBZ/TiO2 films are listed in Table 1. In each nanocomposite film, the content of the benzoxazine monomer is equal, and the different proportions of TiO2 are responsible for forming various rough surfaces. The root-mean-square roughness (Rq) of the PBZ film is only 6.3 ± 0.7 nm which increases with the incorporation of TiO2 into PBZ. The Rq of the PBZT9 film is 134.8 ± 14.1 nm, and the PBZT11 film enjoys a larger Rq of 162.2 ± 22.7 nm, of which the superhydrophobicity is better than that of PBAT9. After further addition of TiO2, e.g. PBZT40, the Rq decreases to 107.3 ± 11.9 nm and the water CA declines to ∼143 ± 4°. Overall, it can be concluded that the CA results are consistent with the surface roughness which is the main factor influencing the wettability of PBZ/TiO2 films.
| Samples | Water CA (degree) | Water CAH (degree) | SA (degree) | Rq(nm) |
|---|---|---|---|---|
| a The polymerized nanocomposite films were denoted as PBZTx, where x is the relative weight of TiO2 (x wt%) to the weight of the benzoxazine monomer, e.g. PBZT10. | ||||
| PBZ | ∼106 ± 1 | ∼29 ± 1 | ∼90 ± 1 | 6.3 ± 0.7 |
| PBZT5a | ∼117 ± 2 | ∼72 ± 1 | — | 82.0 ± 18.4 |
| PBZT9 | ∼123 ± 1 | ∼64 ± 1 | — | 134.8 ± 14.1 |
| PBZT11 | ∼166 ± 1 | ∼3 ± 1 | ∼1 ± 1 | 162.2 ± 22.7 |
| PBZT15 | ∼153 ± 2 | ∼13 ± 1 | ∼10 ± 2 | 142.7 ± 19.9 |
| PBZT40 | ∼143 ± 3 | ∼53 ± 1 | — | 107.3 ± 11.9 |
To realize the wetting phenomenon of PBZ/TiO2 films, we also measured the advancing and receding contact angles by using an increment–decrement method. The water CAH is the value of the advancing contact angle minus the receding contact angle. As listed in Table 1, the water CAH values of superhydrophobic PBZT11 and PBZT15 are ∼3 ± 1° and ∼13 ± 1° respectively as well as having SAs ≤ 10°, which are in accordance with their large water CAs. The CA and SA image of the PBZT11 surface is also shown in Fig. S3.† However, the water CAH values of hydrophobic PBZT5, PBZT9 and PBZT40 films are all larger than 50°. Fig. S4† shows that a water droplet on these surfaces cannot roll down even when the base is tilted 180°, showing a pinning phenomenon. Moreover, as shown in Fig. S3a,† a water droplet on the PBZ film is pinned on the surface when the base is tilted 180°, while it slides down slowly when the base is tilted 90°. The water CAH of PBZ is ∼29 ± 1°.
The results can be further elucidated by applying proper wetting states, described as the Wenzel state or Cassie state, when the content of TiO2 is changed. It has been reported that in the Cassie state drops suspend on top of rough surfaces and in the Wenzel state drops impale on rough surfaces.28,29 When the content of TiO2 is 9 wt%, the film is in the Wenzel state with a hydrophobic property and large water CAH of ∼64 ± 1°. PBZ/TiO2 films accord with the Cassie state when the content of TiO2 is increased to 11 wt%, which exhibits a superhydrophobic property and a minute water CAH of ∼3 ± 1°. Nevertheless, the PBZ/TiO2 film shows hydrophobicity with a large water CAH of ∼53 ± 1° when the content of TiO2 is 40 wt%, which is consistent with the Wenzel state. The wetting phenomenon of PBZ/TiO2 films in the Wenzel state or Cassie state is primarily related to the changes in the surface micro- and nanoscale structures after incorporating TiO2, which causes roughness on the PBZ/TiO2 surface.
The relevant topographic structures of PBZ/TiO2 films with various content of TiO2 are shown in Fig. 2. The PBZT11 film has an apparent rough surface at the micro- and nanoscale which can enhance the surface roughness dramatically and exert a beneficial effect on the superhydrophobicity of a surface. Although with a large content of TiO2 compared to PBZT11, the PBZT40 film has a relatively small surface roughness which is mainly due to the formation of more flat topographical structures on the surface as shown in Fig. 2d. The decreased surface roughness on the PBZT40 surface allows it to be wetted by water, thus showing a hydrophobic surface and conforming with the Wenzel state. Overall, introducing a moderate content of TiO2, from 10% to 20%, can lead to superhydrophobic PBZ/TiO2 films.
Stability and durability of the superhydrophobic PBZ/TiO2 film
For the stability and durability test, the sample PBZT11 was investigated in detail. The superhydrophobic films were heated between 150 °C and 400 °C for 1 h. The variations in hydrophobicity are illustrated in Fig. 3. It is noteworthy that the as-prepared superhydrophobic film maintains a large water CA of ∼160 ± 2° even after 300 °C heat treatment owing to the good thermal stability of the PBZ resin.19 The decrease in the water CA of the PBZT11 film at a higher temperature is mainly due to the cleavage of C–N bonds and the loss of intramolecular bonds in PBZ above 350 °C.19,30 | ||
| Fig. 3 Variations in the water CA of the PBZT11 film at various temperatures. The insets show the corresponding water CA images of PBZT11 at different temperatures. | ||
Furthermore, as shown in Fig. 4a, the superhydrophobic PBZT11 film is a yellowish and transparent film. Since no obvious cracks or defects are observed along the edge of the scratch after the peel-off test shown in Fig. 4b, the PBZT11 film shows strong adhesion to the glass substrate, which can be primarily attributed to the formation of Si–O–Si bonds between the silane-functionalized PBZ and glass substrate.19,31 Finally, the PBZT11 films have been placed under fixed temperature and humidity conditions (25 ± 2 °C, 1 atm with the humidity around 50%) for months. The water CA of the superhydrophobic film after being placed for 6 months was ∼161 ± 3° as shown in Fig. 4c, which demonstrates the environmental durability of the superhydrophobic film. The excellent thermal stability of the superhydrophobic PBZT11 at high temperature with strong adhesion to glass substrates makes it promising for practical applications. Moreover, PBZT11 keeps its superhydrophobicity with a water CA of ∼158 ± 3° after three sandpaper scratch test cycles, demonstrating resistance to mechanical scratching. However, the superhydrophobicity of the PBZT11 surface is lost after the seventh scratch cycle and turns into a hydrophobic surface with a water CA of ∼140 ± 3° as shown in Fig. S5.†
 | ||
| Fig. 4 (a) The appearance of the PBZ/TiO2 films, (b) SEM image of PBZT11 on a glass substrate after the adhesion test, and (c) water CA image of PBZT11 placed for 6 months. | ||
Reversible wettability of PBZ/TiO2 films
The reversibly switchable wettability of PBZ/TiO2 films was investigated through the sequential alternating of UV exposure and heat treatment. As shown in Fig. 5, pure PBZ has no obvious photoresponsive property after 10 hour irradiation and maintains its hydrophobicity, indicating an anti-UV property of the silane-functionalized PBZ. The incorporation of photo-induced superhydrophilic TiO2 NPs into PBZ has an enhancing and controlling effect on the hydrophobicity of the PBZ film under UV irradiation. The water CA of PBZT11 was ∼115 ± 2° when exposed to UV light for 10 h, which is probably due to a large content of PBZ and its non-photoresponsive property. However, the variation in the water CA of each specimen under an equivalent exposure time is enhanced by increasing the content of TiO2. The CAs of PBZT15, PBZT40 and PBZT60 decrease to ∼61 ± 4°, ∼35 ± 7° and ∼26 ± 1° after a 10 hour UV irradiation respectively. In this paper, we chose PBZT60 which exhibits the maximum variation in water CA under UV exposure, to carry out a detailed investigation into the switchable wettability.The hydrophilic PBZT60 film after the first UV exposure can be switched to a hydrophobic one quickly upon heat treatment. It can be concluded from Fig. 6a that the higher the temperature is, the more hydrophobic the film is. Since the curing temperature of PBZ is 200 °C, we choose 200 °C to investigate in detail. As shown in Fig. 6b, the exposed PBZT60 film turns into a hydrophobic film with a water CA of ∼122 ± 1° in only 5 minutes at 200 °C and reaches the highest water CA of ∼128 ± 1° in 30 minutes. The wettability of the PBZT60 film after the first UV exposure and the first heat treatment reveals a reversible transition between hydrophobicity and hydrophilicity. This reversibly switchable wettability can be repeated several times as shown in Fig. 7. The change in the water CA of PBZT60 in each cycle was about 125°. Interestingly, the hydrophobic film can be transformed into a superhydrophilic one with a water CA of 0° after the second UV exposure. This phenomenon is owing to a photocorrosion process of the photo-induced superhydrophilicity of TiO2, and its hydrophilicizing rate increases with repeated UV light irradiation.32
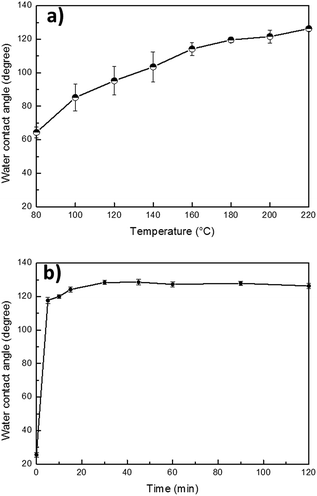 | ||
| Fig. 6 Variations in water CAs of (a) the UV-exposed PBZT60 film at different temperatures, and (b) the UV-exposed PBZT60 film at 200 °C at various times. | ||
 | ||
| Fig. 7 Reversible hydrophobic–superhydrophilic transitions of the PBZT60 film through UV exposure–heat treatment cycles. | ||
These reversible hydrophobic–superhydrophilic transitions are believed to be associated with the content of Ti–OH on the film surface, which is further examined using X-ray photoelectron spectrometer (XPS) analyses. The Ti 2p peaks as well as the curve fitting peaks of Ti–OH (458.7 eV) and Ti–O (459.0 eV) are shown in Fig. 8. On one hand, the area fraction of the Ti–OH peaks increases and that of Ti–O decreases after the first irradiation. On the other hand, the area fraction of the Ti–OH peaks decreases and that of Ti–O increases after the first heat treatment, thereby inducing a hydrophobic PBZ/TiO2 film. This phenomenon is essentially due to an increase in the water adsorption on the surface of the PBZT60 film under UV exposure while there is a decrease in water adsorption under heat treatment. When irradiated by UV light, TiO2 generates electrons and holes.33 Photo-generated holes react with lattice oxygen to form surface oxygen vacancies.34 Then water molecules (hydroxyl groups) are absorbed into the oxygen vacancies leading to water adsorption on the surface, in which this process transforms the originally hydrophobic PBZT60 surface into a superhydrophilic surface. However, the hydroxyl groups are gradually replaced by atmospheric oxygen when the exposed PBZT60 is heated, thus resulting in a hydrophobic surface, which is also consistent with the conclusion in Miyauchi’s report.35 The reversibly switchable PBZ/TiO2 film between highly hydrophobic and superhydrophilic can broaden its application in liquid manipulation.
 | ||
| Fig. 8 XPS spectra (Ti 2p peaks) of (a) the PBZT60 film, (b) the film after the first UV light irradiation for 9 hours, and (c) the film after the first heat treatment at 200 °C for 30 minutes. | ||
Experimental
Materials
4-Methylphenol and 3-aminopropyltrimethoxysilane were purchased from Sinopharm Chemical Reagent Corp. and Shanghai Haiqu Reagent Corp. respectively. Paraformaldehyde, chloroform and calcium hydride were obtained from Shanghai Lingfeng Chemical Corp. Tetrahydrofuran (THF) was purchased from Shanghai Tianlian Fine Chemical Corp. All chemicals were of analytical grade and used as received without further purification, except chloroform which was purified by distillation over calcium hydride. TiO2 NPs (P25) were kindly provided by the Evonik Degussa Specialty Chemicals Company. Their BET surface area is 52.80 m2 g−1. Glass slides (100 × 100 × 1 mm3) obtained from Hongda Medical Equipment Corp. were used as the substrates.Synthesis of the benzoxazine monomer
3-(Trimethoxysilyl)-n-propyl-3,4-dihydro-6-methyl-2H-1,3-benzoxazine monomer (MP-aptms) was synthesized according to the literature.19 A mixture of paraformaldehyde (0.1 mol of formaldehyde) and calcium hydride (7.0 g) in chloroform (50 mL) was added to a 250 mL four-neck flask. The system was heated to 65 °C in an oil bath. Subsequently, 8.95 g of 3-aminopropyltrimethoxysilane was mixed into the system with vigorous stirring upon further heating to 85 °C. Then 4-methylphenol (0.05 mol) in 20 mL of chloroform was added to the flask drop by drop. It continued to react for 3 h. The products were filtered and distilled out to obtain the benzoxazine monomer. The obtained MP-aptms was yellowish and viscous. The weight was 13.4 g and the yield was 89.7%.Preparation of the PBZ/TiO2 nanocomposite films
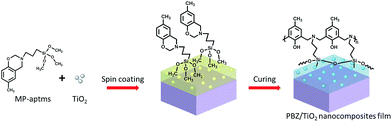
Prior to spin coating, the glass substrates were cleaned ultrasonically in water, ethanol and acetone for 15 min respectively. These substrates were blown dry with nitrogen gas to ensure a dust-free surface after removing the spare acetone through ultrasonication in water again for 5 min. The clean pristine glass substrate is hydrophilic with a water CA of ∼42 ± 1°. Subsequently, the MP-aptms benzoxazine monomer (0.50 g) with a concentration of 10 wt% was dissolved in THF and a certain amount of TiO2 was added to the MP-aptms solution. After holding the mixture in an ultrasound bath for 2 h to form a uniform dispersion, the mixture was spin-coated onto a pre-treated substrate at 1500 rpm for 30 s. Finally, the films were dried in a vacuum oven at 60 °C for 1 h and cured at 200 °C for 1 h since the silane-functionalized PBZ film had the lowest surface free energy at the curing temperature of 200 °C for 1 h. The procedure is illustrated in Scheme 1. The polymerized nanocomposite films were denoted as PBZTx, where x is the relative weight of TiO2 (x wt%) to the weight of benzoxazine monomer, e.g. PBZT10.Stability and durability measurements
To measure the stability of the surface superhydrophobicity, the hydrophobicities of the as-prepared films heated at various temperatures between 150 °C and 400 °C for 1 h were examined. The environmental durability was investigated by analyzing the hydrophobicities of the films which were simply placed indoors with a humidity around 50% for 6 months. The durability measurement was still conducted each month. The adhesive property was measured by a 90° peel-off test.36 Moreover, the sandpaper abrasion test was carried out to measure the mechanical durability of the as-prepared superhydrophobic film according to the literature.1 In detail, a sample weighing 100 g was placed face-down on the sandpaper (grit no. 240) and moved for 10 cm along a ruler. Then the sample was rotated by 90° (facing the sandpaper) and then moved for 10 cm along the ruler. This process is defined as one abrasion cycle.Switchable wettability
The switchable wettabilities of the PBZ/TiO2 films were investigated upon UV exposure and heat treatment. The UV light source was obtained from a 250 W Hg lamp at 25 ± 1.5 °C with a humidity of 60 ± 2%.Characterization
The chemical structures of MP-aptms and poly(MP-aptms) were confirmed by infrared spectroscopic measurements recorded with a Nicolet iS10 FTIR spectrometer in the range of 4000–400 cm−1 at room temperature. A solution of the benzoxazine monomer was directly coated onto a KBr plate and formed a film by drying the solvent. To form the poly(MP-aptms) film, the as-prepared MP-aptms monomer film on the KBr plate was further cured at 200 °C for 1 h. The results are listed in the ESI.† A Data Physics OCA20 optical goniometer interfaced with image-capture software was used to measure the water CAs of the PBZ/TiO2 films at room temperature. A deionized water droplet of 3 μL was injected onto the composite film surface. The advancing and receding contact angles were measured by using a increment decrement method and the corresponding water contact angle hysteresis (CAH) was calculated. The deionized water drops were inflated with an injection rate of 1 μL s−1 and deflated with a suction rate of 1 μL s−1. Then, sliding angle (SA) measurements were carried out with deionized water droplets of 10 μL by a contact angle OCA20 optical goniometer equipped with a tilting base. Each of the reported CAs, CAH values and SAs represent the average of five measurements at different areas of the film. The surface morphologies of the PBZ/TiO2 films were observed with a Nova Nano SEM 450 field emission scanning electron microscope (FE-SEM) at an acceleration voltage of 3 kV. Each specimen was sputtered with gold at 20 mA for 60 s before the SEM observation. The surface roughness profiles of the films were acquired using a Veeco atomic force microscope (AFM) operated in a tapping mode. Each root-mean-square roughness (Rq) value of the films represents the average of three measurements over scan areas of 5 μm × 5 μm. The chemical composition of the film was characterized using a ESCALAB 250Xi XPS and an Al K Alpha line excitation source with the reference of C 1s at 285.00 eV.Conclusions
Without any fluorine-containing surface modification agents, a superhydrophobic PBZ/TiO2 nanocomposite film with a large water CA of ∼166 ± 1° was prepared by incorporating 11 wt% TiO2 NPs through spin coating and further curing. The superhydrophobicity of the PBZ/TiO2 film is stable even after heat treatment at 300 °C because of the high thermal stability of the PBZ resin, and it is durable for more than half a year. The superhydrophobic film has a strong adhesion to glass substrates due to the cross-linking structures in the nanocomposite system. Moreover, the content of TiO2 affects the reversibly switchable wettabilities of the PBZ/TiO2 films. When the content of TiO2 is increased to 60%, the consequent nanocomposite film exhibits hydrophobicity–superhydrophilicity conversions with a variation of around 125° in water CAs upon UV exposure–heat treatment cycles. The photo-induced superhydrophilicity is owing to an increased content of Ti–OH on the surface of the PBZT60 film. The as-prepared, thermally stable and environmentally durable superhydrophobic PBZ/TiO2 film with a strong adhesion to glass substrates shows potential for practical applications and its photo-responsive property can expand its application areas.Acknowledgements
This work was financially supported by the Nanotech Foundation of Science and Technology Commission of Shanghai Municipality (0652nm001), the Program of Shanghai Subject Chief Scientist (10XD1401500), the Fundamental Research Funds for the Central Universities (WA1514015), and the China Postdoctoral Science Foundation (2015M571509).References
- Y. Lu, S. Sathasivam, J. Song, C. R. Crick, C. J. Carmalt and I. P. Parkin, Science, 2015, 347, 1132–1135 CrossRef CAS PubMed.
- C. Dai, N. Liu, Y. Cao, Y. Chen, F. Lu and L. Feng, Soft Matter, 2014, 10, 8116–8121 RSC.
- Z. Xue, Y. Cao, N. Liu, L. Feng and L. Jiang, J. Mater. Chem. A, 2014, 2, 2445–2457 CAS.
- L. Wen, Y. Tian and L. Jiang, Angew. Chem., Int. Ed. Engl., 2015, 54, 3387–3399 CrossRef CAS PubMed.
- B. Su, M. Li and Q. Lu, Langmuir, 2010, 26, 6048–6052 CrossRef CAS PubMed.
- C. Liu, Microfluid. Nanofluid., 2010, 9, 923–931 CrossRef CAS.
- P. N. Nge, C. I. Rogers and A. T. Woolley, Chem. Rev., 2013, 113, 2550–2583 CrossRef CAS PubMed.
- X. Yao, Y. Song and L. Jiang, Adv. Mater., 2011, 23, 719–734 CrossRef CAS PubMed.
- K. Liu, M. Cao, A. Fujishima and L. Jiang, Chem. Rev., 2014, 19, 10044–10094 CrossRef PubMed.
- L. Chen, W. Wang, B. Su, Y. Wen, C. Li, Y. Zhou, M. Li, X. Shi, H. Du, Y. Song and L. Jiang, ACS Nano, 2014, 8, 744–751 CrossRef CAS PubMed.
- B. Xin and J. Hao, Chem. Soc. Rev., 2010, 39, 769–782 RSC.
- L. Zhang, Z. Zhang and P. Wang, NPG Asia Mater., 2012, 4, 1–7 CrossRef PubMed.
- N. Gao and Y. Yan, Nanoscale, 2012, 4, 2202–2218 RSC.
- S. Barthwal, Y. S. Kim and S. H. Lim, Langmuir, 2013, 29, 11966–11974 CrossRef CAS PubMed.
- S. E. Lee, H. J. Kim, S. H. Lee and D. G. Choi, Langmuir, 2013, 29, 8070–8075 CrossRef CAS PubMed.
- H. Choi, S. Choo, J. Shin, K. Kim and H. Lee, J. Phys. Chem. C, 2013, 117, 24354–24359 CAS.
- H. Dong, Z. Xin, X. Lu and Y. Lv, Polymer, 2011, 52, 1092–1101 CrossRef CAS PubMed.
- L. Qu and Z. Xin, Langmuir, 2011, 27, 8365–8370 CrossRef CAS PubMed.
- J. Liu, X. Lu, Z. Xin and C. Zhou, Langmuir, 2013, 29, 411–416 CrossRef CAS PubMed.
- Y. Wang, C. Wang, P. Tung, S. Kuo, C. Lin, Y. Sheen and F. Chang, Langmuir, 2006, 22, 8289–8292 CrossRef PubMed.
- C. Liao, C. Wang, H. Lin, H. Chou and F. Chang, Langmuir, 2009, 25, 3359–3362 CrossRef CAS.
- T. Zhang, H. Yan, Z. Fang, Y. E, T. Wu and F. Chen, Appl. Surf. Sci., 2014, 309, 218–224 CrossRef CAS PubMed.
- X. Tang, Y. Si, J. Ge, B. Ding, L. Liu, G. Zheng, W. Luo and J. Yu, Nanoscale, 2013, 5, 11657–11664 RSC.
- A. Raza, Y. Si, X. Wang, T. Ren, B. Ding, J. Yu and S. S. Al-Deyab, RSC Adv., 2012, 2, 12804–12811 RSC.
- W. Su and S. Kuo, J. Nanomater., 2013, 2013, 1–12 CrossRef PubMed.
- D. Wang, P. Jiao, J. Wang, Q. Zhang, L. Feng and Z. Yang, J. Appl. Polym. Sci., 2012, 125, 870–875 CrossRef CAS PubMed.
- T. Kamegawa, Y. Shimizu and H. Yamashita, Adv. Mater., 2012, 24, 3697–3700 CrossRef CAS PubMed.
- H. Y. Erbil, Surf. Sci. Rep., 2014, 69, 325–365 CrossRef CAS PubMed.
- K. Seo, M. Kim and D. H. Kim, Korea Aust. Rheol. J., 2013, 25, 175–180 CrossRef PubMed.
- S. Tiptipakorn, S. Damrongsakkul, S. Ando, K. Hemvichian and S. Rimdusit, Polym. Degrad. Stab., 2007, 92, 1265–1278 CrossRef CAS PubMed.
- C. Zhou, X. Lu, Z. Xin, J. Liu and Y. Zhang, Corros. Sci., 2014, 80, 269–275 CrossRef CAS PubMed.
- A. F. Nobuyuki Sakai, T. Watanabe and K. Hashimoto, J. Phys. Chem. B, 2003, 107, 1028–1035 CrossRef.
- J. Schneider, M. Matsuoka, M. Takeuchi, J. Zhang, Y. Horiuchi, M. Anpo and D. W. Bahnemann, Chem. Rev., 2014, 114, 9919–9986 CrossRef CAS PubMed.
- L. Zheng, Q. Liu, L. Xiong, Y. Li, K. Han, W. Liu, K. Tao, S. Yang and J. Xia, Thin Solid Films, 2012, 520, 2776–2780 CrossRef CAS PubMed.
- M. Miyauchi, N. Kieda, S. Hishita, T. Mitsuhashi, A. Nakajima, T. Watanabe and K. Hashimoto, Surf. Sci., 2002, 511, 401–407 CrossRef CAS.
- V. A. Ganesh, S. S. Dinachali, A. S. Nair and S. Ramakrishna, ACS Appl. Mater. Interfaces, 2013, 5, 1527–1532 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis procedure and FTIR spectra of MP-aptms and poly(MP-aptms). Photograph of water droplet on pristine glass substrate, PBZ film and PBZ/TiO2 film. Water CA and SA image of PBZT11 film. Water CA images of PBZ, PBZT5 and PBZT40 films when the base was tilted. Sandpaper abrasion test. See DOI: 10.1039/c5ra06410d |
| This journal is © The Royal Society of Chemistry 2015 |