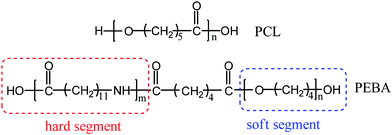
Superior shape memory properties and microstructure evolution of poly(ether-b-amide12) elastomer enhanced by poly(ε-caprolactone)†
Miaoming Huang,
Xia Dong*,
Lili Wang,
Yunyun Gao and
Dujin Wang
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Engineering Plastics, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: xiadong@iccas.ac.cn
First published on 2nd June 2015
Abstract
In this work, the superior shape memory properties and microstructure evolution of poly(ether-b-amide12) (PEBA) enhanced by poly(ε-caprolactone) (PCL) were investigated. The PEBA/PCL blends were prepared by the methods of solution mixing and compression molding. The representative phase-separated morphologies were observed by optical microscopy (OM). The recovery stress and shape memory properties of the samples were determined by dynamic mechanical analysis (DMA). It was found that high loadings of PCL (up to 35 wt%) only marginally worsened the recovery properties of the blends. However, the values of maximum recovery stresses increased greatly with PCL content in spite of deformation temperature, indicating an enhancement effect of PCL on the recovery stress of polymer blends. Moreover, in situ wide angle X-ray scattering (WAXS) investigation revealed the microstructure evolution during the shape memory process. It was notable that during the stretching process, the strain-induced crystallization of soft segments (PTMO) of PEBA shifted to lower strain due to the existence of the PCL phase. The present results provided a reference that the recovery stress could be greatly increased by adding another polymer to the shape memory polymer, which is vital to application in the biomedical field.
1 Introduction
Shape memory polymers (SMPs) can be fixed in a temporary shape and recover to the permanent shape in response to external stimuli such as heat, electric field, water, microwave and magnetic field.1–12 Except for SMPs, some other materials, such as shape memory alloys (SMAs)13,14 and liquid-crystalline elastomers,15–18 also have excellent shape memory effect. Rousseau et al.15 have prepared smectic-C liquid crystalline elastomers and found that the shape fixing and recovery values during the shape memory cycle were respectively about 83.6% and 99.1%, which were competitive in comparison with many SMPs under comparable strains. In addition, SMAs are known for large recovery stress and limited strain, while many SMPs are outstanding in the aspects, such as high recoverable strain, complex shapes, lower density, and some SMPs may be even biodegradable and biocompatible.13,19,20 However, a significant drawback of many SMPs is their relatively small recovery stress under constraint.8 In order to tailor the recovery stress, a number of methods have been tried, including the addition of nanofillers21–24 and deforming SMPs at the proper temperature.25–27 Sun et al.26 found that given a thermo-responsive shape memory polymer, it was possible to alter the recovery stress by means of selecting the programming temperature. Gall et al.21 presented that with the addition of 20 wt% silicon carbide (SiC) in the thermoset epoxy system, 50% increase of constrained bending recovery in the nanocomposites could be achieved. Koerner et al.22 demonstrated that the uniform dispersion of carbon nanotubes (1–5 vol%) in the thermoplastic polyurethane could raise the recovery stress by 50% than the pure resin. Nevertheless, there are two challenges of nanofillers/SMPs composites. Firstly, the uniform dispersion of nanofillers in SMPs matrix is a vital problem, especially for very high loadings of nanofillers. Secondly, the biological evaluation is mandatory prior to their applications as biomaterials. Some types of nanofillers are acutely toxic and the nanofillers/SMPs composites have to be subjected to rigorous assessment before biomedical applications.28–30 Therefore, adding a rigid polymer to the SMPs may be a feasible alternative to the improvement of recovery stress, particularly for the biomedical application.Actually, the mixing of polymers is another way to obtain new materials with satisfactory properties, which has been extensively accepted in the past decades. The architecture of blends, which is of crucial importance to the materials' performance, is not only affected by the crystallization but also controlled by the miscibility of components. For all the polymers, most are totally immiscible, and only a few are partially or completely miscible. The common architectures of immiscible binary polymer blends are cocontinuous and sea-island morphologies. As a simple and controllable way to design new desirable materials, blending has been applied to prepare the shape memory polymer blends, and the morphology–property relationship of which have been extensively studied.31–36 Blends of poly(ε-caprolactone) (PCL) and styrene-butadiene-styrene triblock copolymer (SBS) were reported by Zhang et al.,31 and a general shape memory mechanism for polymer blends with two immiscible components was proposed. It was found that the optimized design of phase morphology contained the elastomer major continuous phase and the crystalline polymer minor continuous phase. Kurahashi et al.34 have investigated the shape memory behavior of thermoplastic polyurethane (PU) and crystalline poly(oxyethylene) (PEO) blends, which could be expressed by a simple mechanical viscoelastic model. Zhao et al.35,36 have also reported the SMPs with triple shape memory effects by chemically cross-linked immiscible blends with cocontinuous architecture.
However, to the best of our knowledge, the detailed study on the recovery stress and microstructure evolution of shape memory polymer blends under constraint have been seldom concerned and studied. Therefore, it is of great importance to design a shape memory system and to in situ monitor the stress-induced mechanical and morphological evolution. In our previous works, we have studied the effects of interaction between polyurethane segments and SiO2 surface on the microstructure evolution of PU nanocomposites,37 and done some research about the effects of gel content of cross-linked poly(ε-caprolactone) on the two-way shape memory property, and the structural origin was also discussed systemically.38 Now in this work, we will further investigate the recovery stress in the blend system to obtain super shape memory property. In view of the above background, poly(ether-b-amide12) copolymer (PEBA) and poly(ε-caprolactone) (PCL) are used as SMP and modifier, respectively. PEBA, a type of commercial thermoplastic elastomers, consists of poly(tetramethylene ether) glycol as the soft segments, polyamide 12 as the hard segments and a diacid acting as the joint between the two segments.39,40 The PEBA possesses a number of excellent performances including biocompatibility, processability and good mechanical properties, endowing the material potential applications in many fields such as food packaging films, gas separation membranes and in the medical applications such as short-term implantation and virus-proof surgical sheeting.41,42 As a semicrystalline polymer with low toxicity, biocompatibility and biodegradability, the PCL can also be used in the biomedical field.43 In particular, the PCL is in a state of rigid crystalline at room temperature, which can therefore be used as a reinforcing modifier for the soft thermoplastic elastomers. Given all these benefits, PCL may be a good blending partner for PEBA to prepare shape memory polymer blends for uses in both commodity and biomedical applications. In this work, PEBA and PCL blends were prepared by the methods of solution mixing and compression molding. The effects of PCL on the recovery stress of PEBA were systematically investigated. More importantly, wide angle X-ray scattering (WAXS) was employed to study the instantaneous microstructure evolution of the samples during the shape memory process under constant strain. The results provide a reference for shape memory polymer to increase the recovery stress dramatically by the addition of another polymer, which is of great importance for the design and applications of such kind of functionalized materials, especially in the biomedical application.
2 Experimental
2.1 Materials
Poly(ε-caprolactone) (PCL, Mn = 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) was purchased from Sigma-Aldrich. The poly(ether-b-amide12) copolymer (PEBA) (Arkema Company) is a copolymer of poly(tetramethylene ether) glycol (PTMO, soft segments) and polyamide 12 (PA12, hard segments). The PEBA 2533, used in this study, has an average-number molar mass of approximately 50
000 g mol−1) was purchased from Sigma-Aldrich. The poly(ether-b-amide12) copolymer (PEBA) (Arkema Company) is a copolymer of poly(tetramethylene ether) glycol (PTMO, soft segments) and polyamide 12 (PA12, hard segments). The PEBA 2533, used in this study, has an average-number molar mass of approximately 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1.44 The degree of polymerization for PA12 blocks (m) and PTMO blocks (n) of this used PEBA are about 3 and 28, respectively.45 Chemical structures of these polymers are illustrated in Scheme 1. N,N-Dimethylacetamide (DMAc) and methanol are of analytical grade. All chemicals were used without any further purification.
000 g mol−1.44 The degree of polymerization for PA12 blocks (m) and PTMO blocks (n) of this used PEBA are about 3 and 28, respectively.45 Chemical structures of these polymers are illustrated in Scheme 1. N,N-Dimethylacetamide (DMAc) and methanol are of analytical grade. All chemicals were used without any further purification.
2.2 Preparation of samples
Prior to solution mixing, both PEBA and PCL were dried at 40 °C under vacuum for 24 h to remove excess moisture. PEBA and PCL blends with various weight ratios were dissolved in DMAc at 110 °C for 2 h, and then coprecipitated into a large excess of methanol. After filtration, the blends were dried in air for 24 h and further dried in a vacuum oven at 40 °C for 3 days. The films of blends were obtained by compression molding at 160 °C for 3 min. All the samples were referred to as follows: 100/0, 80/20, 65/35 and 0/100, where the first and second numbers correspond to the weight percentage of PEBA and PCL in the blends, respectively.2.3 Optical microscopy
The morphology of the samples was studied by an optical microscope equipped with a Linkam hot stage (LTS 350). Phase contrast optical microscopy (PCOM) and polarized optical microscopy (POM) images were obtained by an Olympus (BX51) optical microscope equipped with a Canon 40D camera system. The experimental procedure was as follows: the sample was first annealed at 160 °C for 3 min to erase the thermal history, and then quenched using ice water to observe the crystalline and phase morphology.2.4 Characterization of thermo-mechanical property
The thermal behaviors of the samples were obtained by Perkin-Elmer Diamond DSC. The instrument was calibrated with indium before test. The samples were first cooled to −50 °C at 10 °C min−1, and then heated to 200 °C at 10 °C min−1. The melting temperature (Tm), crystallization temperature (Tc) and the heat of fusion (ΔHm) were evaluated from the two runs.The dynamic-mechanical behaviors of the samples were obtained using Dynamic Mechanical Analysis (DMA Q800, TA Instruments). The tests were performed in the tension mode from −130 °C to 100 °C at 3.0 °C min−1. The strain and frequency were 0.1% and 1 Hz, separately.
2.5 Characterization of shape memory property
The shape memory tests were carried out in a tensile and strain controlled mode on DMA Q800. (1) The sample was stretched at deformation temperature (Td = 0, 10, 20 and 30 °C) by ramping the strain to 100% at rate of 50% min−1. (2) Then the sample was cooled down to the low temperature (Tlow = −20 °C) under the constant applied strain. (3) Finally, the shape fixity ratio and recovery ratio of the sample were measured upon heating from −20 °C to 40 °C under stress-free condition (Fig. 1a), while the recovery stresses of the sample were studied by heating from −20 °C to 60 °C under constant strain recovery condition (Fig. 1b). All tests were performed with the same heating and cooling rate (3 °C min−1).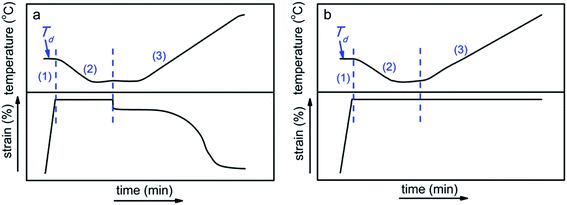 | ||
| Fig. 1 Diagrams of the temperature and strain conditions as a function of time during the shape memory process. The sample was recovered under (a) stress-free and (b) constant strain condition. | ||
The fixity ratio (Rf) and recovery ratio (Rr) of the samples are defined as follows:34
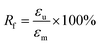 | (1) |
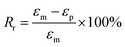 | (2) |
2.6 Microstructure evolution
The microstructure evolution of samples during the shape memory cycle was investigated by in situ WAXS. The tests were performed on the samples with a tensile hot stage (Linkam TST 350). The sample was firstly stretched at Td to a strain of 100%, and then cooled to −20 °C at 3 °C min−1 under fixed strain condition. Finally, the sample was heated to 40 °C at 3 °C min−1 under constant strain condition.WAXS measurements were carried out on the beamline BL16B1 in the Shanghai Synchrotron Radiation Facility (SSRF). The wavelength of the radiation source is 1.24 Å. The WAXS patterns were collected by a MAR 165 detector with a resolution of 2048 × 2048 pixels (pixel size = 79 × 79 μm2). The image acquisition time and the sample-to-detector distance were 17.5 s and 178.36 mm, respectively. All the patterns were corrected for air scattering, background scattering and beam fluctuations.
3 Results and discussion
3.1 Phase morphology
The composition of the blends is one of the most important factors that influence the phase structure. Fig. 2 shows the phase morphologies of the resultant samples that have been melted at 160 °C for 3 min and quenched using ice water. The representative phase-separated morphologies are evidently observed, indicating that the components of PCL and PEBA are immiscible. With 20 wt% PCL, the sample shows distinct sea-island structure, in which the PCL forms isolated phase (white) randomly dispersed in the enriched PEBA matrix phase (black). At a higher content of PCL (35 wt%), a cocontinuous structure is formed, and the PCL domains get evidently bigger, since the immiscibility of the two polymers drive the polymers segregate from each other. Furthermore, even though the PEBA and PCL domains is of very large phase size, it is easy to see that a large amount of very small PEBA phase dispersed randomly in the enriched PCL phase, while a huge number of very small PCL phase also dispersed randomly in the enriched PEBA phase.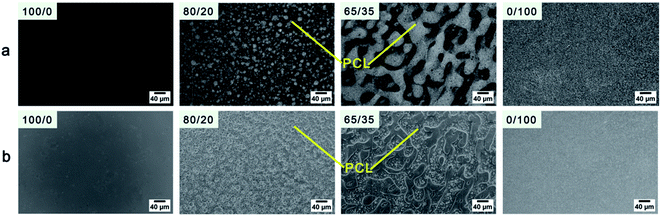 | ||
| Fig. 2 (a) POM and (b) the corresponding PCOM micrographs of the PEBA/PCL samples after annealed at 160 °C for 3 min and quenched using ice water. | ||
3.2 Thermal and mechanical properties
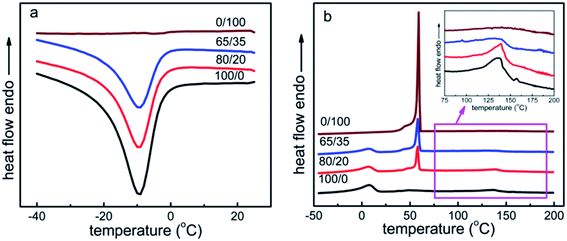
The physical properties of the samples greatly depend on the solid-state morphology, as both the PEBA and PCL are semi-crystalline polymers. Accordingly, DSC and DMA were used to study the original phase transition behaviors of the samples and to make sure that the later tests were performed at suitable temperatures.Fig. 3 presents the typical DSC thermal behaviors of all the samples, and the DSC results are detailedly summarized in Table 1. The pure PEBA (100/0) shows a crystallization peak at about −9.4 °C, which is ascribed to the crystallization of soft segments (PTMO). The crystallization temperature of soft segments in all the samples changes little. However, the half crystallization times (t1/2) of soft segment of PEBA vary from 40.5 s for sample 100/0 to 38.0 s for sample 80/20 and finally to 36.7 s for sample 65/35 (Fig. S1†), revealing that the isothermal crystallization rate of soft segment of PEBA is slightly promoted by the existence of PCL component. The reason may be that the presence of phase interfaces in the samples could reduce the barrier of surface free energy,46 which would benefit the nucleation and crystallization of soft segments. In addition, the melting peaks at around 7 °C and 138 °C are separately attributed to the melting of soft segments and hard segments of PEBA, while the melting peak at about 58 °C corresponds to the melting of PCL. Along with the increase of PCL content up to 35 wt%, the normalized ΔHm1,PTMO in all the samples drops down (Table 1), indicating a decrease of crystallization content for soft segments.
| Samples | 100/0 | 80/20 | 65/35 | 0/100 |
|---|---|---|---|---|
| a Note: the values of ΔHm1,PTMO, ΔHm2,PA and ΔHm,PCL have been normalized, ΔHm1,PTMO = ΔH′m1,PTMO/χPEBA, ΔHm2,PA12 = ΔH′m2,PA12/χPEBA and ΔHm,PCL = ΔH′m,PCL/χPCL (χPEBA and χPCL indicate the weight percentage of PEBA and PCL in the blends). | ||||
| Tm1,PTMO (°C) | 7.7 | 6.8 | 7.1 | — |
| Tm2,PA12 (°C) | 137.7 | 138.1 | 137.9 | — |
| Tc,PTMO (°C) | −9.4 | −9.5 | −9.4 | — |
| ΔHm1,PTMO (J g−1) | 25.4 | 22.3 | 20.5 | — |
| ΔHm2,PA12 (J g−1) | 12.4 | 12.6 | 10.6 | — |
| Tm,PCL (°C) | — | 57.7 | 58.1 | 58.7 |
| ΔHm,PCL (J g−1) | — | 118.5 | 102.3 | 88.2 |
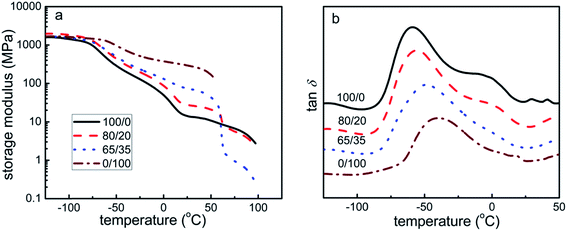
The temperature dependence of storage modulus (E′) and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ for all the samples is shown in Fig. 4. It is evident that the glass transition temperature (Tg) for all the samples range from −60 °C to −40 °C. The temperature of −100 °C is below the Tg of PEBA and PCL, and hence they are in the both crystalline and glassy state. Upon further heating above the Tg of PEBA and PCL, the domains that are crystalline remain solid and immobilized, but the amorphous or glassy polymer chains start to move. At this time, a decrease in E′ is observed for all samples, but the values of E′ increase with the PCL content. On the contrary, the crystalline of PCL melts after 55 °C and the values of E′ decrease with the PCL content. As has been reported in the previous papers,47,48 the E′ of the blends results from the contribution of both PEBA and PCL. When the temperature is above Tg but below the melting temperature of PCL, the PCL is stiffer than the PEBA, leading to higher E′ for samples with higher PCL content. However, after the melt of PCL, and the E′ of the blends is dominated by the PEBA, resulting in the lower E′ for samples with higher PCL content.
δ for all the samples is shown in Fig. 4. It is evident that the glass transition temperature (Tg) for all the samples range from −60 °C to −40 °C. The temperature of −100 °C is below the Tg of PEBA and PCL, and hence they are in the both crystalline and glassy state. Upon further heating above the Tg of PEBA and PCL, the domains that are crystalline remain solid and immobilized, but the amorphous or glassy polymer chains start to move. At this time, a decrease in E′ is observed for all samples, but the values of E′ increase with the PCL content. On the contrary, the crystalline of PCL melts after 55 °C and the values of E′ decrease with the PCL content. As has been reported in the previous papers,47,48 the E′ of the blends results from the contribution of both PEBA and PCL. When the temperature is above Tg but below the melting temperature of PCL, the PCL is stiffer than the PEBA, leading to higher E′ for samples with higher PCL content. However, after the melt of PCL, and the E′ of the blends is dominated by the PEBA, resulting in the lower E′ for samples with higher PCL content.
Taking the results of DSC and DMA into consideration, the later tests were performed at several selective temperatures (between 0 °C and 30 °C).
3.3 Shape memory property
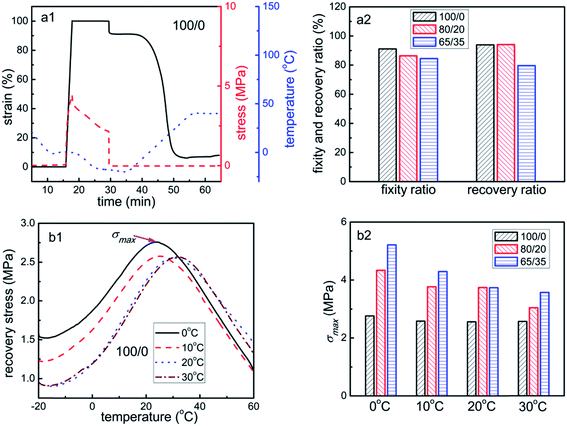
Fig. 5a shows the shape memory cycle of sample 100/0 and the shape memory property data of samples 100/0, 80/20 and 65/35 deformed at 0 °C. The shape memory property of pure PCL (0/100) can't be measured, as it will break before stretched to 100% strain. With the increase of PCL content, the shape fixity ratios (Rf) decrease slightly from 91.1% to 86.4%, and finally to 84.5%, while the shape recovery ratios (Rr) also change from 93.9% to 94.1%, and finally reduce to 79.6%. The reasons about this phenomenon are described as follows in detail. Firstly, according to the theory of Huang and his group,27,49,50 the PEBA itself consists of an elastic segment and a transition segment. Since the PCL is always in the state of crystalline at the deformation temperatures and below (based on the results of Fig. 3 and Table 1), the PCL should be almost regarded as the rigid fillers that can improve the elastic modulus of PEBA greatly, which is evidenced by the fact that the storage modulus of PEBA/PCL between 0 °C and 30 °C increases with the PCL content (Fig. 4a). Therefore, it can be say that the relative contents of transition segment in the PEBA/PCL samples are lower compared with those in the pure PEBA, especially for samples with higher PCL content, which leads to the reduction of Rf with the increase of PCL content. Secondly, for the same overall strain in the material and under the same stretching conditions, the PEBA in PEBA/PCL may experience much higher strain than the pure PEBA. This is attributed to the high stiffness and hence low strains experienced by the PCL,51 which will be discussed and evidenced by in situ WAXS experiments in Section 3.4. As a result, the non-recoverable deformation in both the elastic segment and transition segment of PEBA in the PEBA/PCL may increase, resulting in the lower Rr.The recovery stresses of sample 100/0 and the maximum recovery stresses (σmax) of samples 100/0, 80/20 and 65/35 deformed at different deformation temperature (Td) are presented in Fig. 5b. Despite of Td, the values of σmax increase dramatically with PCL content, indicating an enhancement effect of PCL on the recovery stress of PEBA. For instance, the σmax vary from 2.7 MPa (for sample 100/0) to 4.3 MPa (for sample 80/20) and to 5.2 MPa (for sample 65/35) when deformed at 0 °C, namely that by adding 20 wt% and 35 wt% PCL, the σmax of the samples can be raised by 59.3% and 92.6% than the pure PEBA, respectively. Besides as has been reported previously,23,52 the σmax decreases largely with an increase of Td. This phenomenon can be explained from two perspective. On the one hand, as has been discussed above, the elastic modulus of PEBA is greatly improved by the addition of PCL, and thus higher stress and higher applied mechanical energy are required to stretch the samples 65/35 and 80/20. Since the applied mechanical energy will be transformed to internal energy and stored in the polymer, the stored mechanical energy will increase with the PCL content, which will finally translate into the higher recovery stress during the shape recovery process. On the other hand, when the Td increases from 0 °C to 30 °C, the elastic modulus of PEBA decreases, and the lower applied mechanical energy are needed to stretch the samples, leading to lower stored mechanical energy, and hence lower recovery stress.
From the discussion above, it can be concluded that with damaging the recovery ratio slightly, the materials that have higher recovery stress can be obtained by adding another polymer to SMPs.
3.4 Microstructure evolution during the shape memory process
Fig. 6 shows the WAXS patterns and the corresponding 1D intensity profiles of all the samples at room temperature. The WAXS patterns of all the samples show some evident rings, as typically found for isotropic crystalline orientation. For pure PEBA (100/0), one main peak is observed at 17.1°, corresponding to (001) diffraction of the γ phase of PA12.53,54 Since the melting temperature of PTMO is around 7 °C, it is in the amorphous state at room temperature. The strong amorphous halo at the low angle side is principally due to PTMO phase (soft segments). For pure PCL (0/100), the WAXS patterns show two sharp rings, located at 17.1° and 19.0° and related respectively to the (110) and (200) planes of PCL orthorhombic crystal form.43,55 Additionally, the samples 80/20 and 65/35 evidently show all the diffraction peaks of both polymers. It can be reasonably concluded that PEBA and PCL form their own crystals, respectively.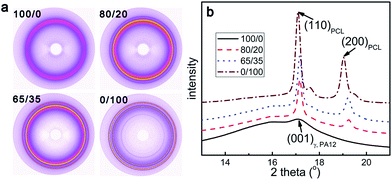 | ||
| Fig. 6 (a) WAXS patterns and (b) the corresponding 1D intensity profiles of the samples 100/0, 80/20, 65/35 and 0/100 at room temperature. | ||
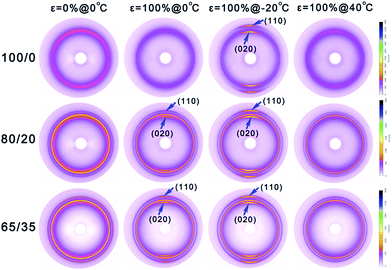
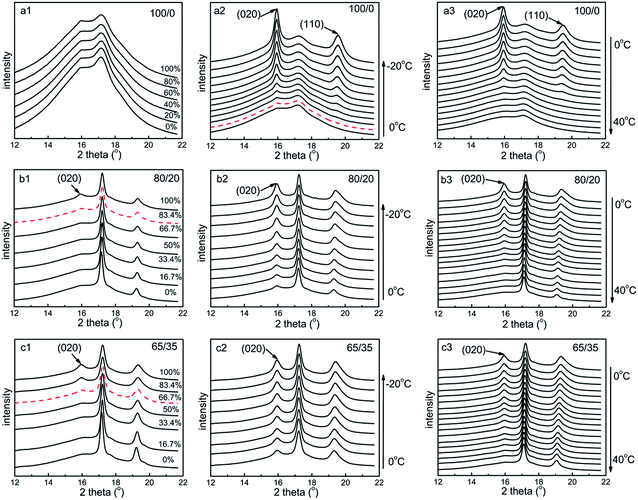
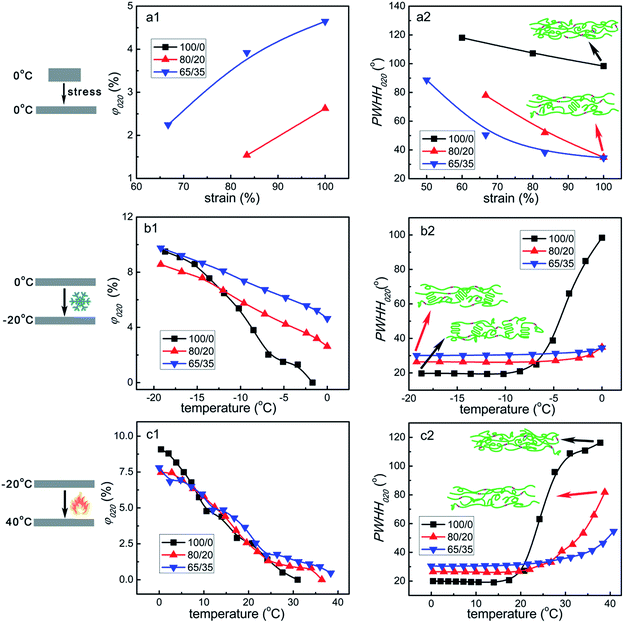
In situ WAXS have been carried out to better understand the microstructure evolution of samples during the shape memory process. Fig. 7 shows the selected 2D WAXS patterns of all the samples stretched to ε = 100% strain at 0 °C and then cooled to −20 °C under constant 100% strain, and finally heated to 40 °C under constant 100% strain. And the corresponding 1D WAXS intensity profiles of all the samples are presented in Fig. 8. To supply a precise evaluation of crystallization and preferred orientation, the relative content of (020) plane reflection of PTMO (φ020) and peak width at half height of the corresponding azimuthal scanning profiles for (020) plane reflection of PTMO (PWHH020) at different strain and temperature are plotted in Fig. 9. At ε = 0% strain, there is no PTMO (or soft segments) crystals at 0 °C and the WAXS patterns are completely isotropic with an amorphous halo in the initial state. At ε = 100% strain, although there is almost no PTMO crystals formed for sample 100/0, the parallel orientation of amorphous PTMO chains is indeed detected. For samples 80/20 and 65/35, two sharp crystalline diffraction peaks emerge on the meridian at ε = 100% strain. One peak overlaps with the amorphous halo, and the other peak appears at the higher angle, which are a signature of the strain-induced crystallization of soft segments.54,56–59 The two peaks can be respectively indexed as (020) and (110) of PTMO crystal.57–60 It is apparent that with the addition of PCL, the formation of PTMO crystals shifts to lower strain and φ020 increase during the stretching process (Fig. 9a1), which can be attributed to two aspects. On the one hand, as has been evidenced by Fig. S1,† the existence of PCL component can slightly promote the crystallization of PTMO. On the other hand, it must be considered that the PCL deforms little, and the PTMO parts of PEBA account for the majority of deformation (Fig. S2†). In view of the existence of PCL component, the magnitude order of effective strain ratio (ε′) of PTMO is ε′65/35 > ε′80/20 > ε′100/0, which has also been found in the stretching process of filled and unfilled natural rubber.61,62 In my opinion, the latter plays a dominant role, as the acceleration effect of PCL on the crystallization rate of PTMO is extremely small. In addition, the order of PWHH020 during the stretching process is PWHH020,65/35 < PWHH020,80/20 < PWHH020,100/0, namely that the order of orientation degree (Φ) of all the samples is Φ65/35 > Φ80/20 > Φ100/0, as the lower value of PWHH is associated with a more oriented structure. More importantly, as the formation of PTMO crystals shifts to lower strain, much more applied mechanical energy is required to continue stretching the already crystallized samples during the stretching process, leading to higher stored mechanical energy and hence higher recovery stress. Therefore, the reinforcement effects of PCL on the recovery stress of PEBA can be attributed to two reasons: acting as rigid fillers and promoting the crystallization of PTMO.
When cooled to −20 °C under constant 100% strain, a large amount of preferentially oriented PTMO crystals form, as presented in Fig. 7, 8(a2, b2, c2) and 9(b1, b2). As the temperature reduces, the φ020 of all the samples goes up, and that of sample 100/0 rises more quickly. Additionally, upon cooling, the orientation degree of sample 100/0 increases much more greatly and rapidly, whereas that of sample 80/20 and 65/35 increases marginally and slowly. It must be noted that contrary to that in the stretching process, the order of final Φ of all the samples during the cooling process is Φ65/35 < Φ80/20 < Φ100/0. The explanation about it could be described as follows. For one thing, the formation of PTMO crystals takes place both during the stretching process (ε ≤ 100% strain) and upon cooling at ε = 100% strain, resulting in a lower and a higher degree of orientation, respectively. For another thing, the φ020 of sample 65/35 formed under the two conditions are ∼4.6% and ∼5.1%, while those of sample 80/20 are ∼2.6% and ∼5.9%. On the contrary, those of sample 100/0 are ∼0% and ∼9.5%. In other words, sample 100/0 has the largest content of PTMO crystals formed upon cooling at ε = 100% strain, leading to the highest degree of orientation.
Upon heating to 40 °C under constant 100% strain, the preferentially oriented PTMO crystals gradually melt. The φ020 of all the samples drops down continually with the temperature. However, the orientation degrees of all the samples almost have no change below 20 °C. Then the orientation degree of sample 100/0 decreases drastically and quickly, while that of samples 80/20 and 65/35 decreases slowly. However, for all the samples, the resultant amorphous PTMO chains after melting still keep a certain extent of parallel orientation to the stretching direction. It is interesting that the sequence of Φ for all the samples is Φ65/35 > Φ80/20 > Φ100/0, which may be attributed to the influence of effective strain ratio (ε′), as the magnitude order of ε′ for PTMO is ε′65/35 > ε′80/20 > ε′100/0.
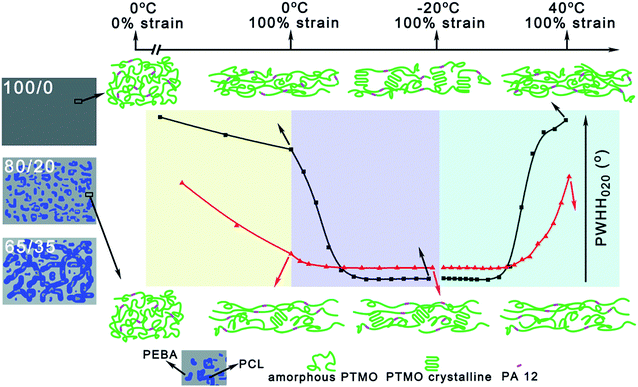
Based on the experimental results discussed above, the microstructure evolution of samples 100/0 and 80/20 during the shape memory process are schematically illustrated in Fig. 10. As the microstructure evolution of sample 65/35 is similar to that of sample 80/20, the sample 65/35 is not included in Fig. 10. Before stretching, the original structure of PEBA components in both samples 100/0 and 80/20 are in the random orientation state. When deformed to 100% strain at 0 °C, the PTMO chains in both 100/0 and 80/20 samples orient along the stretching direction. However, contrary to the occurrence of strain-induced crystallization of PTMO in sample 80/20, no crystallization of PTMO happens in sample 100/0 at 100% strain and 0 °C. Then a lot of oriented PTMO crystals form upon cooling to −20 °C under constant 100% strain, while the relative content and orientation degree of PTMO crystals in sample 100/0 are higher. Finally, the oriented PTMO crystals melt when heated to 40 °C under constant 100% strain. It is noted that the amorphous PTMO chains after melting still exhibit a certain degree of orientation along the stretching direction. At this time, sample 80/20 has a higher degree of orientation than sample 100/0.
4 Conclusions
In this study, the PEBA/PCL blends exhibited the typical phase-separated morphologies and superior shape memory property. The isothermal crystallization rate of soft segments (PTMO) of PEBA was slightly promoted by PCL, which was resulted from the presence of phase interfaces that reduced the barrier of surface free energy. While the recovery ratio of PEBA only decreased marginally with PCL content, the σmax increased dramatically regardless of the Td due to the higher stored mechanical energy, suggesting the reinforcement effect of PCL on the recovery stress of PEBA. In addition, the σmax reduced greatly with the increase of Td, as higher Td would lead to lower stored mechanical energy, and hence lower recovery stress. Moreover, the existence of PCL phase drove the strain-induced crystallization of PTMO appeared at lower strain during the stretching process, leading to the fact that much more applied mechanical energy was required to continue stretching the already crystallized samples, resulting in higher stored mechanical energy and hence higher recovery stress. Therefore, the reinforcement effects of PCL on the recovery stress of PEBA were mainly due to two factors: acting as rigid fillers and promoting the crystallization of PTMO. When cooled to −20 °C under constant 100% strain, a large amount of preferentially oriented PTMO crystals formed. Upon heating to 40 °C under constant 100% strain, the resultant amorphous PTMO chains after melting of all the samples still had a parallel orientation to the stretching direction. It must be noted that the magnitude order of orientation degree for all the samples was Φ65/35 > Φ80/20 > Φ100/0, which was ascribed to the different effective strain ratio of PTMO chains. The most important founding of the present work was that the recovery stress of SMPs could be dramatically improved by the addition of another crystalline polymer, and the microstructure, the morphology evolution and corresponding property of SMPs could be controlled. It is important to help designing and extending applications of complex functionalized SMPs materials, especially in the biomedical applications.Acknowledgements
We would like to thank the generous financial support of the following grants: National Natural Sciences Foundation of China (no. 51173195), R&D Program of the Ministry of Science and Technology (no. 2013BAE02B02) and the beam time on BL16B1 beamline in the Shanghai Synchrotron Radiation Facility (SSRF).References
- Y. Zhu, J. L. Hu, H. S. Luo, R. J. Young, L. B. Deng, S. Zhang, Y. Fan and G. D. Ye, Soft Matter, 2012, 8, 2509 RSC.
- X. Z. Gu and P. T. Mather, RSC Adv., 2013, 3, 15783 RSC.
- L. S. Zhang, Y. H. Jiang, Z. Xiong, X. Q. Liu, H. N. Na, R. Y. Zhang and J. Zhu, J. Mater. Chem. A, 2013, 1, 3263 CAS.
- G. X. Fei, G. Li, L. S. Wu and H. S. Xia, Soft Matter, 2012, 8, 5123 RSC.
- J. Zotzmann, M. Behl, D. Hofmann and A. Lendlein, Adv. Mater., 2010, 22, 3424 CrossRef CAS PubMed.
- G. Li, G. X. Fei, B. Liu, H. S. Xia and Y. Zhao, RSC Adv., 2014, 4, 32701 RSC.
- K. Yu, Y. J. Liu and J. S. Leng, RSC Adv., 2014, 4, 2961 RSC.
- T. Xie, Polymer, 2011, 52, 4985 CrossRef CAS PubMed.
- S. S. Mahapatra, M. S. Ramasamy, H. J. Yoo and J. W. Cho, RSC Adv., 2014, 4, 15146 RSC.
- H. Meng and G. Q. Li, Polymer, 2013, 54, 2199 CrossRef CAS PubMed.
- Y. J. Liu, H. Y. Du, L. W. Liu and J. S. Leng, Smart Mater. Struct., 2014, 23, 023001 CrossRef.
- J. L. Hu, Y. Zhu, H. H. Huang and J. Lu, Prog. Polym. Sci., 2012, 37, 1720 CrossRef CAS PubMed.
- L. Sun, W. M. Huang, Z. Ding, Y. Zhao, C. C. Wang, H. Purnawali and C. Tang, Mater. Des., 2012, 33, 577 CrossRef CAS PubMed.
- A. Lendlein and S. Kelch, Angew. Chem., Int. Ed., 2002, 41, 2034 CrossRef CAS.
- I. A. Rousseau and P. T. Mather, J. Am. Chem. Soc., 2003, 125, 15300 CrossRef CAS PubMed.
- A. Sanchez-Ferrer and H. Finkelmann, Macromol. Rapid Commun., 2011, 32, 309 CrossRef CAS PubMed.
- K. M. Lee, T. J. Bunning and T. J. White, Adv. Mater., 2012, 24, 2839 CrossRef CAS PubMed.
- L. Yu, Z. X. Cheng, Z. J. Dong, Y. H. Zhang and H. F. Yu, J. Mater. Chem. C, 2014, 2, 8501 RSC.
- C. Liu, H. Qin and P. T. Mather, J. Mater. Chem., 2007, 17, 1543 RSC.
- I. A. Rousseau, Polym. Eng. Sci., 2008, 48, 2075 CAS.
- K. Gall, M. L. Dunn, Y. P. Liu, D. Finch, M. Lake and N. A. Munshi, Acta Mater., 2002, 50, 5115 CrossRef CAS.
- H. Koerner, G. Price, N. A. Pearce, M. Alexander and R. A. Vaia, Nat. Mater., 2004, 3, 115 CrossRef CAS PubMed.
- P. Miaudet, A. Derre, M. Maugey, C. Zakri, P. M. Piccione, R. Inoubli and P. Poulin, Science, 2007, 318, 1294 CrossRef CAS PubMed.
- I. S. Gunes, F. Cao and S. C. Jana, Polymer, 2008, 49, 2223 CrossRef CAS PubMed.
- N. Fritzsche and T. Pretsch, Macromolecules, 2014, 47, 5952 CrossRef CAS.
- L. Sun, W. M. Huang, C. C. Wang, Y. Zhao, Z. Ding and H. Purnawali, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 3574 CrossRef CAS PubMed.
- W. M. Huang, Y. Zhao, C. C. Wang, Z. Ding, H. Purnawali, C. Tang and J. L. Zhang, J. Polym. Res., 2012, 19, 9952 CrossRef.
- M. Crosera, M. Bovenzi, G. Maina, G. Adami, C. Zanette, C. Florio and F. F. Larese, Int. Arch. Occup. Environ. Health, 2009, 82, 1043 CrossRef CAS PubMed.
- A. E. Porter, M. Gass, K. Muller, J. N. Skepper, P. A. Midgley and M. Welland, Nat. Nanotechnol., 2007, 2, 713 CrossRef CAS PubMed.
- Y. Xiao, S. B. Zhou, L. Wang and T. Gong, ACS Appl. Mater. Interfaces, 2010, 2, 3506 CAS.
- H. Zhang, H. T. Wang, W. Zhong and Q. G. Du, Polymer, 2009, 50, 1596 CrossRef CAS PubMed.
- X. F. Luo and P. T. Mather, Macromolecules, 2009, 42, 7251 CrossRef CAS.
- S. H. Ajili, N. G. Ebrahimi and M. Soleimani, Acta Biomater., 2009, 5, 1519 CrossRef CAS PubMed.
- E. Kurahashi, H. Sugimoto, E. Nakanishi, K. Nagata and K. Inomata, Soft Matter, 2012, 8, 496 RSC.
- J. Zhao, M. Chen, X. Y. Wang, X. D. Zhao, Z. W. Wang, Z. M. Dang, L. Ma, G. H. Hu and F. H. Chen, ACS Appl. Mater. Interfaces, 2013, 5, 5550 CAS.
- Z. W. Wang, J. Zhao, M. Chen, M. H. Yang, L. Y. Tang, Z. M. Dang, F. H. Chen, M. M. Huang and X. Dong, ACS Appl. Mater. Interfaces, 2014, 6, 20051 CAS.
- M. M. Huang, X. Dong, Y. Y. Gao, Q. Xing, W. L. Li and D. J. Wang, Polymer, 2014, 55, 4289 CrossRef CAS PubMed.
- M. M. Huang, X. Dong, L. L. Wang, J. Zhao, G. M. Liu and D. J. Wang, RSC Adv., 2014, 4, 55483 RSC.
- J. P. Sheth, J. N. Xu and G. L. Wilkes, Polymer, 2003, 44, 743 CrossRef CAS.
- Y. Song, H. Yamamoto and N. Nemoto, Macromolecules, 2004, 37, 6219 CrossRef CAS.
- K. Y. Zhang, V. Nagarajan, M. Misra and A. K. Mohanty, ACS Appl. Mater. Interfaces, 2014, 6, 12436 CAS.
- S. Sridhar, T. M. Aminabhavi, S. J. Mayor and M. Ramakrishna, Ind. Eng. Chem. Res., 2007, 46, 8144 CrossRef CAS.
- T. Kamal, T. J. Shin and S. Y. Park, Macromolecules, 2012, 45, 8752 CrossRef CAS.
- V. I. Bondar, B. D. Freeman and I. Pinnau, J. Polym. Sci., Part B: Polym. Phys., 1999, 37, 2463 CrossRef CAS.
- M. E. Rezac and T. John, Polymer, 1998, 39, 599 CrossRef CAS.
- F. Sakai, K. Nishikawa, Y. Inoue and K. Yazawa, Macromolecules, 2009, 42, 8335 CrossRef CAS.
- A. M. Mihut, A. Sanchez-Ferrer, J. J. Crassous, L. A. Hirschi, R. Mezzenga and H. Dietsch, Polymer, 2013, 54, 4194 CrossRef CAS PubMed.
- J. M. Haberl, A. Sanchez-Ferrer, A. M. Mihut, H. Dietsch, A. M. Hirt and R. Mezzenga, Nanoscale, 2013, 5, 5539 RSC.
- X. L. Wu, W. M. Huang, Y. Zhao, Z. Ding, C. Tang and J. L. Zhang, Polymers, 2013, 5, 1169 CrossRef PubMed.
- L. Sun, W. M. Huang, C. C. Wang, Z. Ding, Y. Zhao, C. Tang and X. Y. Gao, Liq. Cryst., 2014, 41, 277 CrossRef CAS PubMed.
- I. S. Gunes and S. C. Jana, J. Nanosci. Nanotechnol., 2008, 8, 1616 CrossRef CAS PubMed.
- H. B. Lu, Soft Matter, 2013, 9, 11157 RSC.
- L. G. Bai, Z. H. Hong, D. L. Wang, J. J. Li, X. Wang, G. G. Pan, L. B. Li and X. H. Li, Polymer, 2010, 51, 5604 CrossRef CAS PubMed.
- T. Kamal, S. Y. Park, J. H. Park and Y. W. Chang, Macromol. Res., 2012, 20, 725 CrossRef CAS.
- Y. B. Zhang, V. Leblanc-Boily, Y. Zhao and R. E. Prud'homme, Polymer, 2005, 46, 8141 CrossRef CAS PubMed.
- F. Yeh, B. S. Hsiao, B. B. Sauer, S. Michel and H. W. Siesler, Macromolecules, 2003, 36, 1940 CrossRef CAS.
- Y. I. Odarchenko, D. V. Anokhin, D. Doblas, M. Rosenthal, J. J. Hernandez, L. Vidal, N. J. Sijbrandi, A. J. Kimenai, E. P. C. Mes, R. Broos, G. Bar, P. J. Dijkstra, J. Feijen, M. Soloviev and D. A. Ivanov, Macromolecules, 2014, 47, 7890 CrossRef CAS.
- M. C. E. J. Niesten, S. Harkema, E. Van Der Heide and R. J. Gaymans, Polymer, 2001, 42, 1131 CrossRef CAS.
- S. Warner, J. Elastomers Plast., 1990, 22, 166 CrossRef CAS PubMed.
- K. Imada, T. Miyakawa, Y. Chatani, H. Tadokoro and S. Murahash, Makromol. Chem., 1965, 83, 113 CrossRef CAS PubMed.
- S. Poompradub, M. Tosaka, S. Kohjiya, Y. Ikeda, S. Toki, I. Sics and B. S. Hsiao, J. Appl. Phys., 2005, 97, 103529 CrossRef PubMed.
- B. Ozbas, S. Toki, B. S. Hsiao, B. Chu, R. A. Register, I. A. Aksay, R. K. Prud'homme and D. H. Adamson, J. Polym. Sci., Part B: Polym. Phys., 2012, 50, 718 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra06409k |
| This journal is © The Royal Society of Chemistry 2015 |