Anchoring cobalt oxide nanoparticles on to the surface multiwalled carbon nanotubes for improved supercapacitive performances
Babasaheb R. Sankapal*a,
Hemant B. Gajareb,
Swapnil S. Karadea and
Deepak P. Dubalc
aNano Material and Device Laboratory, Department of Applied Physics, Visvesvaraya National Institute of Technology, South Ambazari Road, Nagpur, 440010 Maharashtra, India. E-mail: brsankapal@phy.vnit.ac.in; Fax: +91 712 2223230; Tel: +91 712 2801170
bThin Film and Nano Science Laboratory, Department of Physics, School of Physical Sciences, North Maharashtra University, Jalgaon – 425 001, Maharashtra, India
cCatalan Institute of Nanoscience and Nanotechnology, ICN2 (CSIC-CERCA), Campus UAB, E-08193 Bellaterra, Barcelona, Spain
First published on 22nd May 2015
Abstract
The present work explored a novel, simple and low cost ‘dipping and drying’ process followed by a successive ionic layer adsorption and reaction (SILAR) method for the synthesis of cobalt oxide anchored multiwalled carbon nanotubes (Co3O4/MWNTs). Initially, MWNTs have been coated on a stainless steel substrate by a simple ‘dip and dry’ method, on which further deposition of cobalt oxide nanoparticles was carried out by the SILAR method. Our results confirm the uniform coating of Co3O4 nanoparticles having sizes less than 15 nm on the surface of MWNTs. Later, the electrochemical performance shows that, the Co3O4/MWNTs films exhibit a maximum specific capacitance of 685 F g−1 in a 2 M KOH electrolyte at a scan rate of 5 mV s−1 with high cycle stability of 73% over 5000 cycles. Moreover, lower electrochemical equivalent series resistance (11.25 mΩ) give rise to the superior performance. These results show, the potential of Co3O4/MWNTs composite electrodes in electrochemical supercapacitors.
Introduction
In recent years, research has been focused on enhanced safety, long cycle life, high-power and energy-density storage devices due to the ever-increasing needs in modern electronics industry such as digital communication, short-term power sources for mobile electronic devices, electric vehicles, hybrid electric vehicles, and other power supply facilities.1 In this regard, different batteries and high performance capacitors are the focus of the scientific community.2 Supercapacitors have attracted intense interest owing to their combined merits, i.e., high power density of conventional dielectric capacitors and high energy density of batteries.3 The key to develop supercapacitors is the design and synthesis of high-performance electrode materials. With this understanding, new ideas in electrochemical energy storage devices that are superior to conventional energy storage devices are under challenging investigations. Carbon materials,4 transition metal oxides5 and conducting polymer6 have been proved favourable for supercapacitors because of their high theoretical capacitance.Recently, metal oxide and carbon nanotubes nanocomposites materials have been attracted great attention due to their excellent properties which combines the individual materials property and their synergistic effects for electrode materials.7 The introduction of metal oxides in one-dimensional carbon nanotubes (CNTs) with hollow interior space are ideal candidate due to available more reaction sites with conducting channels. To date, many reports are available in literatures to fabricate the different electrochemical metal oxide/MWNT composites such as NiO/MWNTs,8 MnO2/MWNTs,9 TiO2/MWNTs,10 V2O5/MWNTs11 and Fe3O4/MWNTs.12 From the literature survey, the Co3O4/MWNTs composite as a typical, with the consideration of low cost, natural abundance, environmental safety, good corrosion stability, good long term performance, and low cost have been reported to be promising electrode materials for supercapacitors.13 Cobalt oxide can interact with ions not only at the surface, but also throughout the bulk as well.14 For the past few years, considerable efforts have been focused on cobalt oxide electrode materials for supercapacitors. However, very few reports are available on composite of Co3O4/MWNTs electrodes such as microwave assisted approach, chemical precipitation method, electrophoretic deposition, hydrothermal method, in situ decomposition method, electrostatic co-precipitation and in situ decomposition method.15–17 Amongst these, hydrothermal, Electrostatic co-precipitation, solution method, two step surfactant assisted method which is one of the most popular growth techniques for coating Co3O4. These conventional physical techniques generally produce good quality transparent films. However, these methods do not satisfy the requirements for commercial application because of their complicated multi-step preparation and cost. However, synthesis of Co3O4/MWNTs with unique hybrid nanostructure directly on the support/substrate to use as supercapacitive electrode is still a challenge. On the other hand, chemical deposition techniques are relatively low cost processes and can be easily scaled up for industrial applications. Among the chemical techniques, chemical bath deposition (CBD) is one of the simplest methods of depositing Co3O4.
In this work, we are providing very simple and efficient approach for the synthesis of Co3O4/MWNTs thin film as an electrode for supercapacitors. This approach includes two different steps, (1) coating of MWNTs by dip and dry method and (2) subsequent deposition of Co3O4 nanoparticles on MWNTs by successive ionic layer adsorption and reaction (SILAR) method. Moreover, the work is also aimed to increase the electronic conductivity and stability of Co3O4 nanodots using MWNTs with Co3O4 nanoparticles in the outer shell and highly conducting MWNTs as inner core. Electrochemical supercapacitive properties of Co3O4/MWNTs hybrid electrodes have been systematically investigated.
Results and discussion
Co3O4/MWNTs thin films were prepared by deposition of MWNTs on stainless steel substrates using dip and dry method on which subsequent anchoring of Co3O4 nanoparticles using SILAR method. Briefly, functionalized MWNTs were well dispersed into the solution containing the distilled water and 1 wt% Triton X-100 using an ultrasonicator. During the sonication Triton X-100 molecules get adsorbed on the MWNTs surface, interpreting them soluble in aqueous system. When the substrate was immersed in this solution, the MWNTs were adsorbed onto the substrate due to the attractive forces between the substrate and the MWNTs. These forces may be cohesive, van der Waal's forces or chemical attractive forces. Repetition of such cycles makes uniform and well adherent coating of MWNTs on stainless steel substrate. Further, Co3O4 nanoparticles were anchored on MWNTs using SILAR method. In details, when substrate with pre-coated MWNTs was dipped in cationic precursor, cobalt hexamine complex species ([Co(NH3)6]2+) in the solution get adsorbed on MWNTs due to cohesive/van der Waal's/chemical attractive forces between complexed cobalt ions and the functionalised MWNTs.19| [Co(H2O)6]2+ + 6NH3 → [Co(NH3)6]2+ + 6H2O | (1) |
Later, the immersion of complexed cobalt species decorated MWNTs substrate in aqueous solution of H2O2 (0.1%) results in the reaction between OH− (from aq. H2O2) ions and pre-adsorbed cobalt hexamine complex ions at the surface of MWNTs which at the end forms cobalt oxyhydroxide layer according to the following reaction,
| [Co(NH3)6]2+ + 2H2O2 → CoO(OH) + NH3↑ + 2H2O | (2) |
The cycle of ion adsorption followed by oxidation reaction was repeated several times to achieve terminal thickness of Co3O4/MWNTs film. Finally, the films were annealed for 2 h at 723 K, in order to remove oxyhydroxide phase to pure oxide.
 | (3) |
CoO(OH) is very reactive with O2, and forms a higher oxide Co3O4. Thus, the uniform and well-adherent Co3O4/MWNTs thin films were obtained on the stainless steel substrates.
XRD patterns are recorded for bare steel, MWNTs, and Co3O4/MWNTs thin film and presented in Fig. 1(a). The characteristic of graphitic (002) peak of the MWNTs at the 2θ = 25.3° was clearly observed in both spectra of MWNTs and Co3O4/MWNTs samples. In addition to the characteristics peak, the reflections along (220) and (440) obtained at 49.20° and 64.30° corresponds to Co3O4 material [JCPDS 70-0816]. Other peaks obtained at 43.66, 50.32, 65.21, and 74.46° results from the contribution of the stainless steel substrate. Since the stainless steel peaks are much stronger than Co3O4 peaks, the weak peaks of Co3O4 are overlapped. There were no other impurity peaks observed in XRD analysis.
 | ||
| Fig. 1 (a) XRD patterns of the bare stainless steel, MWNTs and Co3O4/MWNTs thin film (b) FTIR spectra of MWNTs and Co3O4/MWNTs samples. | ||
FTIR is used to analyse the chemical bonding and type of functional groups grafted onto the MWNTs. FTIR spectra of functionalised MWNTs and Co3O4/MWNTs samples are shown in Fig. 1(b). As seen from the spectra, functionalized MWNTs exhibits some prominent absorption bands at 1653, 1511, 1219 cm−1, respectively, which are originated from the graphitic component of MWNTs. The absorption peak can be found at 1653 cm−1 due to the attachment of carboxyl group onto the surface of MWNTs. The appearance of two strong bands at 626 and 575 cm−1 are attributed to the vibration of Co–O in the Co3O4/MWNTs sample which is a clear evidence of the presence of Co3O4.20
Fig. 2(a and b) show FESEM images of Co3O4/MWNTs films at two different magnifications. It is seen that, the outside surface of MWNTs is uniformly anchored with Co3O4 nanoparticles. No aggregation of Co3O4 was seen in the porous area between MWNTs network, indicating that the nucleation occurs predominantly on the outer surfaces of MWNTs and just some micrometres long MWNTs are uniformly entangled to form uniform three dimensional interconnected networks. Close inspection of Co3O4/MWNTs confirms the uniformity of nanoparticles less than about 15 nm in average size.
Detailed information about the size, shape and distribution Co3O4/MWNTs were obtained from transmission electron microscopy (TEM) images. Low magnified image (Fig. 3(a)) shows, the porous and uniform distribution of Co3O4 particle, which is consistent with the FESEM image. It is also seen that, Co3O4 nanoparticles are anchored without showing any aggregation and sustaining porous nature of MWNTs. Because growth occurs through an ion by ion mechanism in SILAR method, no aggregation of Co3O4 nanoparticles occurs on the walls of the MWNTs. Moreover, high magnified image (Fig. 3(b)) revealed the formation of nanoparticles of about less than 15 nm. Some big nanoparticles are observed which might be due to aggregation of nanoparticles. The interplaner spacing is calculated to be 0.33 nm (Fig. 3(c)), which corresponds to the (002) plane in MWNTs and 0.27 nm from Fig. 3(d), which corresponds to the (220) plane of Co3O4, respectively. In addition, the selected area electron diffraction (SAED) was performed to investigate the crystalline characteristics of Co3O4/MWNTs thin film (see inset of Fig. 3(d)). The SAED pattern of Co3O4/MWNTs thin film reveals polycrystalline nature. Thus, the lattice fringes in HRTEM image and the selected area electron diffraction (SAED) pattern further confirmed the formation of crystalline Co3O4.
Cyclic voltammetry (CV) was performed to investigate supercapacitive performance of Co3O4/MWNTs electrodes in KOH electrolyte within a potential window of 0.2 to +0.5 V (vs., Ag/AgCl). In order to find suitable electrolyte concentration for good electrochemical performance CV curves of Co3O4/MWNTs composite were recorded at 100 mV s−1 scan rate at different concentrations of KOH (see Fig. 4(a)). The effect of concentration of KOH electrolyte was studied by keeping the scan rate and potential window constant. CV curves were more distorted with low concentration and did not show well-resolved redox peaks. As concentration of the electrolyte was increased from 0.1 to 2.0 M, the redox peaks were seen distinctly. This change in capacitance may be attributed to the presence of Co3O4 in the composite electrode leading to the contribution of pseudocapacitance with double layer capacitance of MWNTs. This concludes that pseudo-capacitive contribution is dominating than the double layer capacitance.
The specific capacitance of the samples was estimated from the integrated charge from the CV data using the equation;
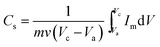 | (4) |
Fig. 4(c) shows CV curves of Co3O4/MWNTs thin film at different scan rates from 5 to 100 mV s−1. It is obvious that the shape of the CV curves does not change evidently even at higher scan rates and the total current density increases with increasing scan rates, which reveals a good rate property and excellent capacitance behaviour for Co3O4/MWNTs electrode. Further, it is clear that voltammetric currents are directly proportional to the scan rate of CV, indicating ideal capacitance behaviour.23 It can also be seen that when scan rate is increased, the current density increases rapidly and the separation between the reduction and oxidation peaks becomes larger, which is mainly due to the polarization of the cell under the relatively high scan rate. The specific capacitance values are decreased from 685 to 105 F g−1 with scan rate from 5 mV s−1 to 100 mV s−1 (see Fig. 4(d)). The excellent specific capacitance obtained would be due to fact that the surface morphology of the deposited films significantly affects the capacitance of an electrochemical capacitor. It is further seen that the specific capacitance decreases with increase in scan rate. The decrease in capacitance with the scan rate is attributed to the presence of inner active sites, which cannot precede the redox transitions completely at higher scan rates of CV, probably due to the diffusion effect of cations within the electrode. The higher the scan rate, lower the diffusivity of cations into the bulk of the electroactive material. Henceforth, only the outer surface of Co3O4/MWNTs thin film electrode would be utilized to generate pseudocapacitance.24 At lower scan rates, all the active areas, including external and internal surfaces, can be utilized for charge/discharge and electrochemical utilization of Co3O4 nanoparticles (full utilization of the electrode materials).25
Fig. 5(a) shows the galvanostatic charge/discharge curves of MWNTs, Co3O4 and Co3O4/MWNTs thin films at current density of 2 A g−1. Almost linear relationship between voltage and charging/discharging time for MWNT has been observed which is common phenomenon expected from non-faradic electrodes. However, the charge/discharge curves for Co3O4 and Co3O4/MWNT are not linear suggesting contribution from pseudo-capacitive charge storing mechanism. The specific capacitance (Cs) can be calculated by using the relation:
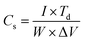 | (5) |
| Material | Method | Electrolyte | Specific capacitance (F g−1) | Ref. |
|---|---|---|---|---|
| Co3O4/CNTs | Solution method | 1 M KOH | 201 (10 mV s−1) | 27 |
| Co3O4/SWNTs | Electrostatic co-precipitation | 6 M KOH | 343 (5 mV s−1) | 28 |
| Co3O4/reduced graphene oxide | Hydrothermal | 2 M KOH | 263 (2 A g−1) | 29 |
| Co3O4/reduced graphene oxide | Two-step surfactant-assisted | 6 M KOH | 159 (5 mV s−1) | 30 |
| Co3O4/reduced graphene oxide | Hydrothermal | 2 M KOH | 472 (2 mV s−1) | 31 |
| Co3O4/MWNTs | Dip and dry followed by SILAR | 2 M KOH | 685 (5 mV s−1) | Present work |
| Co3O4/graphene | Solution based method | 2 M KOH | 478 (5 mV s−1) | 32 |
| Co3O4/MWCNT | Co-precipitation | 2 M KOH | 418 (0.625 A g−1) | 33 |
| Co3O4/graphene | Microwave assisted | 6 M KOH | 243.2 (10 mV s−1) | 34 |
| Co3O4/graphene | Hydrothermal | 2 M KOH | 157.7 (0.1 A g−1) | 35 |
| Co3O4/MWCNT | Hydrothermal | 0.5 M KOH | 590 (15 A g−1) | 36 |
The stability of the electrode in a long period is a significant parameter for evaluating the performance of the electrode. Fig. 6(a) shows the long cycling life test of Co3O4/MWNTs thin film over 1000 cycles at scan rate of 200 V s−1. From the inset of Fig. 6(a), it is seen that all the curves are overlapping each other which specifies good cycling stability. The specific capacitance decreases by small amount after 5000 cycles. It is interesting that the capacity retention of Co3O4/MWNTs electrode after 5000 cycles is about 73%. Moreover, the CV cycling test of the Co3O4/MWNTs film suggests that the synergetic interaction between the MWNTs and Co3O4 significantly improved the electrical properties and the mechanical stability of the electrode.
In order to evaluate the resistances contributed during the charge storing in Co3O4/MWNTs electrode electrochemical impedance measurements were performed at a frequency range from 100 kHz to 0.01 Hz. EIS plot of a supercapacitor device usually divided into three segments following three processes; (i) the bulk resistance of the device or ESR, at high frequencies (>1 kHz); (ii) capacitive effects at high and intermediate frequencies (0.1 kHz); and Warburg diffusion resulting from the frequency dependence of ion diffusion/transport in the electrolyte at low frequencies (<0.1 Hz).26 Fig. 6(b) shows Nyquist plot of Co3O4/MWNTs electrode in 2 M KOH electrolyte. It is seen that, the charge transfer resistance and equivalent series resistance (ESR) (as shown inset) were obtained 11.73 and 0.4, respectively. A low ESR means higher power density as the power density of a supercapacitor is equal to the V2/4Rsm. This low ESR would increase the power density of the electrode.
Conclusions
It is concluded that, we have presented simple and efficient way to anchor cobalt oxide nanodots on to the multiwalled carbon nanotubes in order to fabricate Co3O4/MWNTs nanocomposite for supercapacitor application. Two simple solution based method such as “dip and dry” to coat MWNTs and “SILAR” to anchor Co3O4 nanodots have been successfully exploited. Obtained results confirm the uniform coating of Co3O4 nanodots on the surface of MWNTs. Later, the electrochemical performance shows that Co3O4/MWNTs hybrid electrode exhibits a maximum specific capacitance of 685 F g−1 in 2 M KOH electrolyte at scan rate of 5 mV s−1 with high cycle stability of 73% over 5000 cycles. Moreover, the maximum specific energy obtained for Co3O4/MWNTs electrode was 16.41 W h kg−1 at specific power of 300 W kg−1 which retained to 12.2 W h kg−1 at specific power of 1000 W kg−1 after increasing current density to 5 A g−1. The excellent electrochemical performance is attributed to the synergistic effect of two components and fast redox reactions due to short and diffusion path of ions and electrons. This easy synthesis and short production process would be helpful in production of Co3O4/MWNTs nanostructure on a large scale.Experimental details
Synthesis of Co3O4/MWNTs thin films
To remove amorphous carbon and generate functional groups, MWNTs were refluxed in H2O2 at 60 °C h for suitable time. These functionalised MWNTs are thoroughly washed with double distilled water and then dried in oven at 90 °C for 12 h. Further, 0.125 g of functionalised MWNTs was sonicated in 25 ml aqueous solution of 1 wt% Triton X-100 for 1 h to obtain stable dispersion. Well cleaned stainless steel substrates were dipped in MWNTs solution for 10 min so that the MWNTs get adsorbed on the surface of substrate. The process was repeated by 15–20 times to deposits MWNTs onto substrate. Further these MWNTs coated stainless steel substrate was dried under IR lamps to evaporate the solvents as described previously.18 This stainless steel substrate coated with MWNTs was used as substrate for further deposition of Co3O4 nanodots.Later Co3O4 nanodots were anchored on MWNTs by SILAR method. Briefly, 0.4 M CoCl2, was dissolved in double distilled water; to this complexing agent liquor ammonia was added with constant stirring to maintain the pH ∼ 12 which served as cationic precursor. The oxidant solution of 0.1% H2O2 in double distilled water which served as anionic precursor. Stainless steel substrate with pre-coated MWNTs was dipped in cationic precursor for 40 s, in which complexed cobalt species get adsorbed onto the walls of the MWNTs because of the electrostatics forces of attraction. In next step, the substrate was rinsed with double-distilled water for 15 s to remove loosely bounded or excess complexed cobalt species from the MWNTs coated substrate. Later, the substrate was immersed in anionic precursor (0.1% H2O2) solution for 20 s where the oxygen ions reacts with pre-adsorbed complexed cobalt species on the MWNTs to form cobalt hydroxide film. In the last step, the substrate was again rinsed with double-distilled water for 15 s to remove loosely bounded OH− ions. Thus one cycle for deposition of cobalt hydroxide onto the MWNTs/SS is completed. The cycle of ion adsorption followed by oxidation reaction was repeated several times to increase the thickness of cobalt hydroxide film. Further, the films were annealed at 723 K for 2 h, in order to convert hydroxide phase to pure oxide and also to improve the mechanical stability and electrical conductivity of the electrode and then used for further characterizations.
Characterization techniques
The amount of Co3O4 decorated on MWNTs was measured by commonly used weight difference method using sensitive microbalance. The phase identification of the as-prepared Co3O4/MWNTs nanocomposite was carried out by X-ray Diffractometer (XRD, D8 Advanced, Bruker AXS) with Cu Kα radiation (λ = 1.5418 Å) operating at 40 kV and 40 mA. FTIR spectroscopy analysis was carried out using FTIR spectrometer (JASCO 410) in the spectral range 4000–400 cm−1. The surface morphological study was performed by a field emission scanning electron microscope (FE-SEM, JSM-6360LA) and a high resolution transmission electron microscope (HRTEM, JEOL JEM 2100 Japan, resolution 1.4 Å, AC voltage 200 kV). The electrochemical properties were performed by using potentiostat (Princeton Applied Research, PARSTAT-4000). A typical three electrode cell was employed as a reference electrode (Ag/AgCl), a counter electrode (Platinum wire) and working electrode (Co3O4/MWNTs thin film). All electrochemical experiments were carried out at room temperature. Galvanostatic charge/discharge and electrochemical impedance measurements were performed using the Biologic VMP3 potentiostat. Potentiostat/Galvanostat with impedance software (Princeton Applied Research, PARSTAT-4000).Acknowledgements
BRS is thankful to SERB, Govt. of India through sanctioned project (Do. no: SB/S2/CMP/032/2013, dated 20/12/2013).References
- (a) D. Cericola and R. Koetz, Electrochim. Acta, 2012, 72, 1 CrossRef CAS PubMed; (b) M. Li, J. P. Cheng, J. H. Fang, Y. Yang, F. Liu and X. B. Zhang, Electrochim. Acta, 2014, 134, 309 CrossRef CAS PubMed; (c) C. D. Lokhande, D. P. Dubal and O. S. Joo, Curr. Appl. Phys., 2011, 11, 255 CrossRef PubMed.
- (a) D. P. Dubal, O. Ayyad, V. Ruiz and P. Gomez-Romero, Chem. Soc. Rev., 2015, 44, 1777 RSC; (b) F. Meng and Y. Ding, Adv. Mater., 2011, 23, 4098 CrossRef CAS PubMed.
- (a) Y. F. Yan, Q. L. Cheng, Z. J. Zhu, V. Pavlinek, P. Saha and C. Z. Li, J. Power Sources, 2013, 240, 544 CrossRef CAS PubMed; (b) J. Suarez-Guevara, V. Ruiz and P. Gomez-Romero, J. Mater. Chem. A, 2014, 2, 1014 RSC.
- (a) J. Tang, J. Liu, N. L. Torad, T. Kimura and Y. Yamauchi, Nano Today, 2014, 9, 305 CrossRef CAS PubMed; (b) N. L. Torad, R. R. Salunkhe, Y. Li, H. Hamoudi, M. Imura, Y. Sakka, C. C. Hu and Y. Yamauchi, Chem.–Eur. J., 2014, 20, 7895 CrossRef CAS PubMed.
- (a) X. C. Dong, H. Xu, X. W. Wang, Y. X. Huang, M. B. C. Park, H. Zhang, L. Wang, W. Huang and P. Chen, ACS Nano, 2012, 6, 3206 CrossRef CAS PubMed; (b) Y. F. Yan, Q. L. Cheng, Z. J. Zhu, V. Pavlinek, P. Saha and C. Z. Li, J. Power Sources, 2013, 240, 544 CrossRef CAS PubMed; (c) D. P. Dubal, P. Gomez-Romero, B. R. Sankapal and R. Holze, Nano Energy, 2015, 11, 377 CrossRef CAS PubMed.
- (a) D. P. Dubal, S. H. Lee, J. G. Kim, W. B. Kim and C. D. Lokhande, J. Mater. Chem., 2012, 22, 3044 RSC; (b) R. R Salunkhe, S. H. Hsu, K. CW Wu and Y. Yamauchi, ChemSusChem, 2014, 7, 1551 CrossRef PubMed.
- (a) D. P. Dubal, J. G. Kim, Y. Kim, R. Holze, C. D. Lokhande and W. B. Kim, Energy Technol., 2014, 2, 325 CrossRef PubMed; (b) Y. Huang, J. Tao, W. Meng, M. Zhu, Y. Huang, Y. Fu, Y. Gao and C. Zhi, Nano Energy, 2015, 11, 518 CrossRef CAS PubMed.
- (a) H. Y. Chang, H. C. Chang and K. Y. Lee, Vacuum, 2013, 87, 164 CrossRef CAS PubMed; (b) G. S. Gund, D. P. Dubal, S. S. Shinde and C. D. Lokhande, ACS Appl. Mater. Interfaces, 2014, 6, 3176 CrossRef CAS PubMed.
- S. G. Hwang, S. H. Ryu, S. R. Yun, J. M. Ko, K. M. Kim and K. S. Ryu, Mater. Chem. Phys., 2011, 130, 507 CrossRef CAS PubMed.
- H. I. Kim, H. J. Kim, M. Morita and S. G. Park, Bull. Korean Chem. Soc., 2010, 31, 423 CrossRef CAS.
- Z. Chen, V. Augustyn, J. Wen, Y. Zhang, M. Shen, B. Dunn and Y. Lu, Adv. Mater., 2011, 2, 791 CrossRef PubMed.
- (a) A. K. Mishra and S. Ramaprabhu, J. Phys. Chem. C, 2010, 114, 2583 CrossRef CAS; (b) W. Meng, W. Chen, L. Zhao, Y. Huang, M. Zhu, Y. Huang, Y. Fu, F. Geng, J. Yu, X. Chen and C. Zhi, Nano Energy, 2014, 8, 133 CrossRef CAS PubMed.
- Y. G. Zhu, Y. Wang, Y. Shi and H. Y. Yang, Nano Energy, 2014, 3, 46 CrossRef CAS PubMed.
- S. L. Xiong, C. Z. Yuan, X. G. Zhang, B. J. Xi and Y. T. Quin, Chem.–Eur. J., 2009, 15, 5320 CrossRef CAS PubMed.
- M. Mazloumi, S. Shadmehr, Y. Rangom, L. Nazar and X. Tang, ACS Nano, 2013, 5, 4281 CrossRef PubMed.
- J. Lang, X. Yan and Q. Xue, J. Power Sources, 2011, 196, 7841 CrossRef CAS PubMed.
- H. Fang, S. Zhang, W. Liu, Z. Du, X. Wu and Y. Xing, Electrochim. Acta, 2013, 108, 651 CrossRef CAS PubMed.
- B. R. Sankapal, H. B. Gajare, D. P. Dubal, R. B. Gore, R. R. Salunke and H. Ahn, Chem. Eng. J., 2014, 247, 103 CrossRef CAS PubMed.
- S. G. Kandalkar, J. L. Gunjakar and C. D. Lokhande, Appl. Surf. Sci., 2008, 254, 5540 CrossRef CAS PubMed.
- (a) S. M. Abbas, S. T. Hussain, S. Ali, N. Ahmad, N. Ali and K. S. Munawar, Electrochim. Acta, 2013, 105, 481 CrossRef CAS PubMed; (b) C. K. Dong, X. Li, Y. Zhang, J. Y. Qi and Y. F. Yuan, Chem. Res. Chin. Univ., 2009, 25, 936 CAS.
- (a) T. P. Gujar, V. R. Shinde, C. D. Lokhande and S. Han, J. Power Sources, 2006, 161, 1479 CrossRef CAS PubMed; (b) K. Tsay, L. Zhang and J. Zhang, Electrochim. Acta, 2012, 60, 428 CrossRef CAS PubMed.
- (a) J. P. Zheng and T. R. Jow, J. Electrochem. Soc., 1997, 144, 2417 CrossRef CAS PubMed; (b) B. E. Conway and W. G. Pell, J. Power Sources, 2002, 105, 169 CrossRef CAS.
- (a) D. P. Dubal, V. J. Fulari and C. D. Lokande, Microporous Mesoporous Mater., 2012, 151, 511 CrossRef CAS PubMed; (b) D. P. Dubal, R. Holze and P. Gomez-Romero, Sci. Rep., 2014, 7, 7349 CrossRef PubMed.
- (a) V. Subramanian, H. Zhu and B. Wei, J. Power Sources, 2006, 159, 361 CrossRef CAS PubMed; (b) M. Zhu, W. Meng, Y. Huang, Y. Huang and C. Zhi, ACS Appl. Mater. Interfaces, 2014, 6, 18901 CrossRef CAS PubMed.
- G. S. Gund, D. P. Dubal, S. B. Jambure, S. S. Shinde and C. D. Lokhande, J. Mater. Chem. A, 2013, 1, 4793 CAS.
- (a) G. Yu, L. Hu, M. Vosgueritchian, H. Wang, X. Xie, J. R. McDonough, X. Cui, Y. Cui and Z. Bao, Nano Lett., 2011, 11, 2905 CrossRef CAS PubMed; (b) P. Sen, Electrochim. Acta, 2010, 55, 1 CrossRef PubMed.
- Y. Shan and L. Gao, Mater. Chem. Phys., 2007, 103, 206 CrossRef CAS PubMed.
- A. Abdolmaleki, H. Kazerooni, M. B. Gholivand, H. Heydari and A. Pendashteh, Ionics, 2015, 21, 515 CrossRef CAS PubMed.
- G. J. Liu, L. Q. Fan, F. D. Yu, J. H. Wu, L. Liu, Z. Y. Qiu and Q. Liu, J. Mater. Sci., 2013, 48, 8463 CrossRef CAS.
- W. Zhou, J. Liu, T. Chen, K. S. Tan, X. Jia, Z. Luo, C. Cong, H. Yang, C. M. Li and T. Yu, Phys. Chem. Chem. Phys., 2011, 13, 14462 RSC.
- C. Xiang, M. Li, M. Zhi, A. Manivannan and N. Wu, J. Power Sources, 2013, 226, 65 CrossRef CAS PubMed.
- B. Wang, Y. Wang, J. Park, H. Ahn and G. Wang, J. Alloys Compd., 2011, 509, 7778 CrossRef CAS PubMed.
- J. Lang, X. Yan and Q. Xue, J. Power Sources, 2011, 196, 7841 CrossRef CAS PubMed.
- J. Yan, T. Wei, W. Qiao, B. Shao, Q. Zhao, L. Zhang and Z. Fan, Electrochim. Acta, 2010, 55, 6973 CrossRef CAS PubMed.
- Q. Guan, J. Cheng, B. Wang, W. Ni, G. Gu, X. Li, L. Huang and G. Yang, ACS Appl. Mater. Interfaces, 2014, 6, 7626 CAS.
- X. Wang, M. Li, Z. Chang, Y. Yang, Y. Wu and X. Liu, ACS Appl. Mater. Interfaces, 2015, 7, 2280 CAS.
| This journal is © The Royal Society of Chemistry 2015 |





