Hierarchical Fe3O4@titanate microspheres with superior removal capability for water treatment: in situ growth and structure tailoring via hydrothermal assisted etching†
Liang Shia,
Bohua Dong*b,
Rongjie Gaob,
Ge Sub,
Wei Liub,
Chenghui Xiaac,
Fenghuan Zhaob and
Lixin Cao*b
aCollege of Chemistry and Chemical Engineering, Ocean University of China, Qingdao 266100, PR China
bInstitute of Materials Science and Engineering, Ocean University of China, Qingdao 266100, PR China. E-mail: caolixin@ouc.edu.cn; dongbohua@ouc.edu.cn
cDebye Institute, Utrecht University, Princetonplein 1, 3584 CC Utrecht, Netherlands
First published on 24th August 2015
Abstract
Hierarchical flower-like Fe3O4@titanate microspheres with ultrathin nanosheets-assembled shell were fabricated via an effective step-by-step approach. Superparamagnetic Fe3O4 microspheres pre-formed were used as templates for perfect deposition of the following coatings. Silica middle layer was introduced by Stöber method in order to direct the formation of amorphous titania coverage via the hydrolysis of tetraisopropyl titanate. Under alkaline hydrothermal environment, titanate nanosheets crystallized and grew in situ attaching on the surface of Fe3O4 spheres, generating the flower-like microspheres which exhibit excellent adsorption properties. Meanwhile, the hierarchical structures can be tailored by varying the hydrothermal temperature and alkalinity, and the roles of sodium hydroxide and hydrogen peroxide were proposed.
Introduction
One-dimensional titanate nanostructures constructed via a modified Kasuga's method1 have attracted much attention in recent years for their potential applications, such as catalysis,2 hydrogen storage,3 as sensors4 and as adsorbents.5 Due to the lamellar structure with abundant confined sodium ions, titanate nanostructures are also prevalent in Li-ion batteries.6 Meanwhile, the non-toxicity makes titanate ideal for use as carriers in bio-applications, for example, protein separation.7 Unfortunately, the tendency of these one-dimensional structures to form bundles or random aggregates via an uncontrollable hydrothermal process was inevitable, limiting the optimal performance. As a consequence, researchers are focusing their attention on fabricating hierarchical structures assembled from one-dimensional titanate blocks, generating better capabilities, especially in the field of Li-batteries8 and DSSCs.9Many attempts have been made to fabricate the hierarchical structures, where SiO2 or some colloidal particles are often used as templates.10 Multifunctional hierarchical structures would be simultaneously obtained if the templates were replaced by specific functional particles. Magnetic materials, Fe3O4 for example, have attracted much attention due to their selective separation, controllable drug delivery and nanocatalyst recycling.11 Coincidentally, not only does hierarchical titanate match these potential applications quite well, but also Fe3O4 microspheres synthesized via the solvothermal approach12–14 are natural templates for their regular shape. Many studies have been conducted to achieve multifunctional materials using spherical Fe3O4 as templates, but they still remain at the stage of core–shell structures with simple treatment. Therefore, controllable synthesis and structure tailoring based on magnetic titanates to form hierarchical structures still remain formidable challenges.
Several hierarchical titanate or titania structures combined with magnetic components have been prepared using various strategies. Xuan et al.15 obtained magnetic Fe3O4/TiO2 hollow spheres by using PSA as templates, and the spheres exhibited good photocatalytic activity towards RhB, while the template-induced synthesis seemed complicated and the magnetization was quite weak due to small Fe3O4 particles. Zhao and co-workers16 reported a facile route to synthesize magnetic yolk–shell titanate microspheres, but the preparation was time-consuming and required extreme concentrated basic conditions. Likewise, the work reported by Che et al.17 proposed the microwave-adsorption application of hierarchical Fe3O4@TiO2 yolk–shell microspheres, but the process was also conducted at high temperature and long period.
In this work, we develop a facile strategy to synthesize hierarchical nanostructure based on in situ growth and hydrothermal assisted etching. Firstly, superparamagnetic Fe3O4 microspheres were prepared via the solvothermal method. In order to obtain a firm and dense coverage of amorphous titania, silica was pre-coated and then spherical-like and small steric tetraisopropyl titanate was employed. Finally, hierarchical Fe3O4@titanate microspheres were fabricated by a hydrothermal process in an extremely short reaction period and low alkaline concentration, using hydrogen peroxide as etching agent. The key experiment variables including sodium hydroxide and hydrogen peroxide for morphology controlling have been systematically investigated. The as-synthesized hierarchical Fe3O4@titanate microspheres with uniform size, tailored shell structure, high surface area and large magnetization exhibited excellent water treatment performance with high removal capacities towards organic dyes. The Fe3O4@titanate microspheres could remove almost 85% of the methylene blue within 1 min at room temperature, and adsorption equilibrium reached within 10 min. Furthermore, successful access to these rational designed hierarchical microspheres will make it possible for their potential applications in energy storage, catalysis, and sensing.
Experimental
Chemicals
Poly(4-styrenesulfonic acid-co-maleic acid) sodium salt (PSSMA, SS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA = 1
MA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Mw ∼ 20
1, Mw ∼ 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), titanium isopropoxide (TTIP, 95%), and sodium acetate anhydrous were purchased from Aladdin Chemical Reagent Co. Iron(III) chloride hexahydrate, potassium chloride, sodium hydroxide, ethylene glycol, tetraethyl orthosilicate (TEOS), ethanol, ammonium hydroxide (25–28 wt%) and hydrogen peroxide aqueous solution (≥30%) were all analytical grade and purchased from Sinopharm Chemical Reagent Co., Ltd. The ultra-purity water used in the experiment was produced by Water Purification System (GWA-UN1-F20, Beijing Purkinje General Instrument Co., Ltd).
000), titanium isopropoxide (TTIP, 95%), and sodium acetate anhydrous were purchased from Aladdin Chemical Reagent Co. Iron(III) chloride hexahydrate, potassium chloride, sodium hydroxide, ethylene glycol, tetraethyl orthosilicate (TEOS), ethanol, ammonium hydroxide (25–28 wt%) and hydrogen peroxide aqueous solution (≥30%) were all analytical grade and purchased from Sinopharm Chemical Reagent Co., Ltd. The ultra-purity water used in the experiment was produced by Water Purification System (GWA-UN1-F20, Beijing Purkinje General Instrument Co., Ltd).
Synthesis of superparamagnetic Fe3O4 microspheres
The highly water-soluble and negatively charged superparamagnetic Fe3O4 microspheres were synthesized via hydrothermal method reported by Gao et al.18 Briefly, 0.5 g of PSSMA was dissolved in ethylene glycol (20 mL) under magnetic stirring to form a transparent solution, followed by the addition of FeCl3·6H2O (0.54 g). When a homogeneous red brown solution was obtained, sodium acetate (1.5 g) was then added. The obtained colloidal precursors were transferred and sealed into a Teflon-lined stainless-steel autoclave (50 mL) and heated at 200 °C for 10 h. After reaction and cooling down to ambient temperature, the black products were isolated by a magnet and washed for five times with deionized water, and then dried in vacuum for 12 h.Synthesis of Fe3O4@SiO2 microspheres
The core–shell Fe3O4@SiO2 microspheres were prepared via traditional Stöber method19 as follows. The as-prepared Fe3O4 microspheres (150 mg) were dispersed in absolute ethanol (95 mL) contained in a three-neck round-bottom flask under ultrasound for 15 min. Afterward, 21.6 mL of deionized water and 585 μL of concentrated ammonia were dropped into the solution under mechanical stirring. Finally, 3.33 mL of TEOS was pre-dissolved in 5 mL of ethanol, and mixture was slowly added into the flask in three equal lots. After the reaction was proceeded for 18 h under continuous stirring, the resultant Fe3O4@SiO2 microspheres were washed by ethanol and deionized water for 3 times, respectively. The Fe3O4@SiO2 microspheres were redispersed in 30 mL of ethanol for further use.Amorphous titania coating
The Fe3O4@SiO2 microspheres coated with amorphous titania were fabricated based on the controlled hydrolysis and condensation of TTIP.20 One tens of the above dispersion were mixed with 92 mL of ethanol in a round-bottom flask, and the whole dispersion was ultrasonically treated for 15 min. Afterward, the flask was embedded in ice-bath and the dispersion was stirred mechanically. The addition of 80 μL of KCl (0.1 M) aqueous solution was executed when the dispersion reached steady temperature. After the water component was fully mixed in the dispersion, 320 μL of TTIP dissolved in 5 mL of ethanol were slowly added during 60 min. Then, the reaction was last for another 5 h. The resultants were washed with ethanol for 3 times and denoted as Fe3O4@SiO2@AT.Fabrication of Fe3O4@titanate hierarchical microspheres
The Fe3O4@titanate microspheres were synthesized via an alkaline hydrothermal method.21 The above obtained Fe3O4@SiO2@AT microspheres were fully mixed with an aqueous NaOH solution (0.1 M, 20 mL), then 300 μL of aqueous H2O2 solution (3 wt%) was added into the mixture. Afterward, the mixture was transferred and sealed into a Teflon-lined stainless-steel autoclave (25 mL) and heated at 140 °C for 60 min. After the reaction, the autoclave was quickly cooled down and the products were washed with deionized water for 5 times.Characterization
X-ray diffraction (XRD) experiments were performed with a BRUKER D8 ADVANCE X-ray diffractometer fitted with CuKα radiation over the 2θ ranges from 5° to 80°, and the scanning speed was 4° min−1. TEM images and selected area electron diffraction (SAED) patterns of the samples were obtained on a transmission electron microscope (JEM-1200EX, JEOL) operating at 100 kV. Scanning electron microscope (SEM) inspection and element analysis were conducted using a JSM-6700F (JEOL) equipped with an Oxford INCA energy dispersive X-ray (EDX) analyzer. Fourier transform infrared (FTIR) spectra were collected with Nicolet 6700 spectrometer in the wavelength range of 4000–400 cm−1 at 4 cm−1 resolution. Zeta potential measurements were obtained at ambient temperature using a Zetasizer Nano ZS (Malvern) instrument. Magnetization measurements were performed on a MPMS-XL-7 (Quantum Design) superconducting quantum interference device (SQUID) magnetometer at 300 K.Application as adsorbents
Methylene blue solutions with various initial concentration (10, 20, 30, 40, 60, 80 and 100 mg L−1) were used as probe to evaluate the adsorption properties of hierarchical Fe3O4@titanate. Samples (5 mg) were added into a vial with volume of 10 mL methylene blue solution for the experiment. Every 200 μL of aliquots were taken out and diluted to 2 mL for concentration analysis at each sampling point, and the whole experimental process was conducted in a dark chamber. Sodium hydroxide solution (10 mL, 0.1 M) was used to regenerate the adsorbents, and then the Fe3O4@titanate was washed with water until pH of the filtrate reached neutral. The adsorption properties of as-prepared Fe3O4 microspheres were also tested.Results and discussion
The magnetite particles were synthesized via a solvothermal method reported previously. The obtained Fe3O4 particles own uniformly spherical morphology and an average diameter of ∼198 nm, as shown in Fig. 1(a) and (b). The Fe3O4 spheres exhibit excellent dispersibility in water, because the surface of Fe3O4 spheres was linked with highly negatively-charged macromolecular PSSMA as the reference indicated. The zeta potential of as-prepared Fe3O4 spheres was −52.0 mV (Fig. S1†), which also demonstrated a good dispersibility, favouring the subsequent coating procedures. By using the Stöber method, a smooth silica layer about 10 nm can be easily coated on the surface of as-prepared Fe3O4 spheres, as shown in Fig. 1(c) and (d). On the contrary, the Fe3O4 surface was rough because the sub-microspheres were formed by the aggregation of many small nanocrystals with sizes of 5–8 nm. Moreover, the silica coatings are full of Si–OH groups, which are benefit to amorphous titania deposition.22 Afterwards, the Fe3O4@SiO2 microspheres were coated with a layer of amorphous TiO2 through the traditional sol–gel process. Herein, tetraisopropyl titanate was used as precursor in the hydrolysis for its appropriate hydrolysis rate in neutral water without acidic or basic catalysts, which are both harmful to the existed Fe3O4 or SiO2 components. In addition, the spherical-like and smaller steric tetraisopropyl groups are likely to produce more compacted and uniform titania layer. It can be clearly seen in Fig. 1(e) and (f) that titania layer was successfully deposited on the surface of Fe3O4@SiO2 microspheres, indicating a mean thickness about 20 nm and a rough outer surface once again. The EDX results (Fig. S2†) confirmed the content of Fe, Si and Ti were 34.13, 10.39 and 5.79 by weight, also suggesting the formation of Fe3O4@SiO2@AT core–double shell microstructure. After the Fe3O4@SiO2@AT microspheres were hydrothermally treated, flower-like microspheres with excellent dispersibility (Fig. S3† zeta potential: −34.8 mV) were obtained, as shown in Fig. 1(g). A bumpy shell composed by numerous nanosheets can be clearly identified, while the silica interlayer was hard to be observed by SEM or TEM. However, the EDX results (Fig. S4†) confirmed there was 5.99% Si element by weight, indicating the silica interlayer was not completely removed in the hydrothermal process. Furthermore, these nanosheets possess curled edges and extend to as far as about 50 nm, indicating the entire size of the flower-like microspheres about 300 nm, as revealed in Fig. 1(h).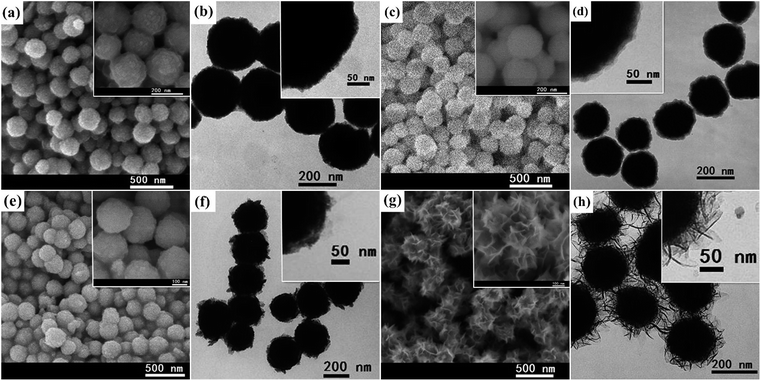 | ||
| Fig. 1 SEM and TEM images of the products at each step: (a) and (b) Fe3O4 microspheres (c) and (d) Fe3O4@SiO2 (e) and (f) Fe3O4@SiO2@AT (g) and (h) Fe3O4@titanate. | ||
XRD was used to investigate the phase composition of the samples at different stages in the entire process. Fig. 2(a) shows the XRD patterns of as-prepared Fe3O4 microspheres, and the diffraction peaks at 2θ = 30.09°, 35.42°, 43.05°, 53.39°, 56.94°, 62.52° and 73.95° can be indexed as (220), (311), (400), (422), (511), (440) and (533) planes of magnetite (JPCDS 19-0629). The XRD patterns of Fe3O4@SiO2 and Fe3O4@SiO2@AT present similar characteristics as their patent Fe3O4, except for a broad peak at 2θ = 25°, which is assigned to amorphous silica, as shown in Fig. 2(b) and (c). However, the situation was quite different for Fe3O4@titanate microspheres when hydrothermal process was conducted. As Fig. 2(d) indicated, a strong peak at 9.7° and small one at 48.0° can be attributed to lepidocrocite-type titanate,23 while the feature of magnetite was also reserved.
 | ||
| Fig. 2 XRD patterns of the products at each step: (a) Fe3O4 microspheres (b) Fe3O4@SiO2 (c) Fe3O4@SiO2@AT (d) Fe3O4@titanate. | ||
As the synthesis procedure involved the formation of several amorphous structures, FTIR analysis was performed to characterize the sequential coating and growth process. Fig. 3 displays the FTIR spectra of corresponding products in the range of 400–4000 cm−1. As shown in Fig. 3(a), the strong band at 574 cm−1 is due to characteristic absorption peaks from the Fe–O in Fe3O4, while bands at 1403 and 1564 cm−1 are assigned to symmetric and asymmetric stretching vibration of carboxylate groups, respectively, indicating the Fe3O4 nanoparticles are bonded with PSSMA. Moreover, the stretching mode of –CH2– (2930 cm−1), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1700 cm−1) and aromatic ring C
O (1700 cm−1) and aromatic ring C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (1453 cm−1) can be observed, also suggesting the existence of PSSMA reserved in the microspheres. As silica layer was coated on the surface of Fe3O4 microspheres, the high-intensity Si–O–Si asymmetric stretching band (1093 cm−1) was observed while the Fe–O bands (574 cm−1) weakened, as revealed in Fig. 3(b). Besides, the additional bands at 475, and 799 cm−1, which are associated with Si–O–Si or O–Si–O bending mode and Si–O–Si symmetric stretching vibration, respectively, can be also detected. After the hydrolysis of tetraisopropyl titanate was completed, the FTIR spectra was quite similar to that of Fe3O4@SiO2, as shown in Fig. 3(c). However, a new band at 947 cm−1 can be found which is attributed to stretching vibration of Ti–O–Si, demonstrating amorphous titania indeed covered the surface of SiO2. Fig. 3(d) displays the spectra of hierarchical microspheres, and characteristics of SiO2 weaken in the hydrothermal process due to silica is susceptible to basic conditions, but still exist as mentioned above. Meanwhile, the broad band centred at 3400 cm−1 was assigned to O–H stretching vibration of physically absorbed water.
C (1453 cm−1) can be observed, also suggesting the existence of PSSMA reserved in the microspheres. As silica layer was coated on the surface of Fe3O4 microspheres, the high-intensity Si–O–Si asymmetric stretching band (1093 cm−1) was observed while the Fe–O bands (574 cm−1) weakened, as revealed in Fig. 3(b). Besides, the additional bands at 475, and 799 cm−1, which are associated with Si–O–Si or O–Si–O bending mode and Si–O–Si symmetric stretching vibration, respectively, can be also detected. After the hydrolysis of tetraisopropyl titanate was completed, the FTIR spectra was quite similar to that of Fe3O4@SiO2, as shown in Fig. 3(c). However, a new band at 947 cm−1 can be found which is attributed to stretching vibration of Ti–O–Si, demonstrating amorphous titania indeed covered the surface of SiO2. Fig. 3(d) displays the spectra of hierarchical microspheres, and characteristics of SiO2 weaken in the hydrothermal process due to silica is susceptible to basic conditions, but still exist as mentioned above. Meanwhile, the broad band centred at 3400 cm−1 was assigned to O–H stretching vibration of physically absorbed water.
 | ||
| Fig. 3 FTIR spectra of the products at each step: (a) Fe3O4 microspheres (b) Fe3O4@SiO2 (c) Fe3O4@SiO2@AT (d) Fe3O4@titanate. | ||
In order to investigate the effects of hydrothermal temperature on the etching process and structure of the final products, we conducted a series of experiments with temperature range of 100–200 °C. Fig. 4(a) shows bare Fe3O4 microspheres and dislocated Ti-fragments were obtained when hydrothermal process was conducted at a quite low temperature (100 °C). A plausible explanation was that the crystallization of amorphous titania was extremely slow while silica coating was dissolved in alkaline condition,24 and titania skin was peeled off into environment in the form of partially crystallized fragments. It can be seen from Fig. 4(b) and (c) that flower-like Fe3O4@titanate microspheres were formed when hydrothermal temperature was increased to 120 °C and 140 °C, but there were still some dislocated components at 120 °C because of slower reaction. However, some nanoparticles were observed attaching to titanate nanosheets as temperature was further increased to 160, as illustrated in Fig. 4(d). At the same time, we can also identify the nanoparticles from TEM images in Fig. 4(e) when temperature was 180 °C. The phenomenon can be interpreted as Fe3O4 primary particle self-assembled in the microspheres fell off under harshly etching at higher temperature. Moreover, titanate nanosheets became fewer and straighter due to a faster reaction rate. When the hydrothermal temperature was set as high as 200 °C, no flower-like products were obtained, as revealed in Fig. 4(f). The amorphous titania coating crystallized and grew at such a high rate that adjacent nanosheets interacted with each other, yielding a disordered shell.
 | ||
| Fig. 4 TEM images of final products synthesized by different hydrothermal temperature for 60 min (a) 100 °C (b) 120 °C (c) 140 °C (d) 160 °C (e) 180 °C (f) 200 °C. In these cases, c(NaOH) = 0.1 M. | ||
To understand the role of sodium hydroxide in the formation of titanate nanosheets, a control experiment was also conducted by varying the concentration of sodium hydroxide aqueous solution. As shown in Fig. 5(a)–(c), various morphologies of final products were obtained by adjusting c(NaOH) to 0 M, 0.1 M and 0.5 M, respectively. As demonstrated by previous research,21 hydrogen peroxide only facilitates curling the titanate nanosheets, and has no effects on the formation of the nanosheets. Herein, hydroxide peroxide was added in all the experiments and the concentration was fixed. When no sodium hydroxide was introduced, bare Fe3O4 microspheres together with some aggregates of nanoparticles were observed, no sheet-like structures were found, as shown in Fig. 5(a). Interestingly, the role of sodium hydroxide and hydrogen peroxide can be realized by this single experiment. As no sodium hydroxide was in the reaction and the silica–titania double-shell was peeled off yet, hydrogen peroxide can be identified as etching agent. Meanwhile, no attached or dislocated sheet-like products were obtained, proving sodium hydroxide plays significant role in formation of titanate nanosheets, as testified by XRD measurements. Moreover, the role of sodium hydroxide can be further certified because longer nanosheets formed at higher concentration, as Fig. 5(c) revealed. Under a relative sufficient concentration of reactant, the Ostwald ripening process was likely to be trigged to form much large and sparse nanosheets, instead of relatively small and dense ones.
 | ||
| Fig. 5 TEM images of obtained products synthesized at 140 °C for 60 min with various concentration of sodium hydroxide (a) 0 M (b) 0.1 M (c) 0.5 M. | ||
Recently, many investigations show that contaminants in freshwater could be partially removed by nano-adsorbents or nanocatalysts25,26 Fe3O4 microspheres were previously demonstrated as good adsorbents for azo-dyes, and can be recovered efficiently through facile magnetic separation.27 Inspired by the hierarchical structure and magnetic properties, we expect the Fe3O4@titanate microspheres possess better performance as recyclable adsorbents. Fig. 6 shows the adsorption plots of methylene blue by as-prepared Fe3O4, Fe3O4@titanate microspheres (140 °C, 0.1 M NaOH) and regenerated materials, respectively. Surprisingly, the Fe3O4@titanate microspheres could remove almost 85% of the methylene blue within 1 min at room temperature, and adsorption equilibrium reached within 10 min. Moreover, the regenerated hierarchical Fe3O4@titanate microspheres could also absorb more than 80% of the methylene blue, while the new as-prepared Fe3O4 reached 70% only. Methylene blue is cationic dye and the electrostatic attractions play significant role in adsorption process. As the as-prepared Fe3O4 microspheres benefit from more negative surface (−52.0 mV vs. −34.8 mV for Fe3O4@titanate), then the excellent Fe3O4@titanate microspheres adsorbents were attributed to the hierarchical structures.
In order to investigate the adsorption capacity of the hierarchical Fe3O4@titanate microspheres, the equilibrium adsorption experiments were conducted by varying initial MB concentrations. As shown in Fig. 7, the adsorption capacity for MB was monotonously increased when the initial concentration was lower than 60 mg L−1. However, the equilibrium adsorption capacity tended to be saturated as the initial MB concentration was further increased. Two equations, Langmuir and Freundlich isotherms models, were employed to analyze the adsorption data. As inset of Fig. 7 shows, the adsorption behaviour of Fe3O4@titanate could be described by Langmuir model, while the experimental data deviated from the Freundlich equation, indicating a monolayer adsorption process of MB by Fe3O4@titanate. As methylene blue is a cationic dye, there exist electrostatic attraction between MB and negatively charged Fe3O4@titanate microspheres, and the adsorbed MB molecules have a repulsive force on the others. Moreover, the maximum adsorption capacity can be calculated by the slope of the linearized Langmuir isotherm (R2 = 0.99918), which was up to 108.11 mg g−1.
 | ||
| Fig. 7 Adsorption isotherm of MB by fresh-prepared Fe3O4@titanate microspheres. Inset: linearized Langmuir isotherm for MB adsorption. | ||
Table 1 lists a comparison of maximum adsorption capacities of magnetic adsorbents for MB removal previously reported. It can be seen that Fe3O4@titanate microspheres have higher adsorption ability than most of those in other literatures. Although the adsorption capacity was smaller than that of previous work by Cui,31 which showed a capacity of 526.3 mg g−1 of MB by Fe3O4–graphene oxide composites, the robust titanate seems to be easier to recycle and can be expanded to apply in catalysis as owning that potential mentioned above.
According to previous report,32 the adsorption kinetics process of methylene blue by titanate conformed to the pseudo-second-order model. Fig. 8 shows the linearized plots of the adsorption process, and plots by partial data were also displayed in the inset as the actual adsorption equilibrium reached within 10 min. As both of the plots exhibited excellent linearity, the adsorption kinetics behaviour of the hierarchical Fe3O4@titanate microspheres fitted the pseudo-second-order model, and the apparent rate (k2 = 4.541) can be calculated from the intercept.
 | ||
| Fig. 8 Linearized pseudo-second-order kinetics model of MB by fresh-prepared Fe3O4@titanate microspheres (initial MB concentration: 30 mg L−1). | ||
Conclusions
We have demonstrated an in situ growth and hydrothermal assisted etching strategy to fabricate flower-like Fe3O4@titanate microspheres with nanosheets-assembled shell. The obtained hierarchical structure reserves the superparamagnetic characteristics, possesses promising application as adsorbents and can be tailored by varying temperature or alkalinity. The preparation procedure is free of organic directing agents and high alkalinity, while accompanied by a time-saving hydrothermal process. This ideal synthetic strategy is expected valid to direct to design other hierarchical composites.Acknowledgements
This research was supported by the National Natural Science Foundation of China (no. 51172218, 51372234, 21301187).Notes and references
- T. Kasuga, M. Hiramatsu, A. Hoson, T. Sekino and K. Niihara, Langmuir, 1998, 14, 3160–3163 CrossRef CAS.
- M. Kitano, K. Nakajima, J. N. Kondo, S. Hayashi and M. Hara, J. Am. Chem. Soc., 2010, 132, 6622–6623 CrossRef CAS PubMed.
- D. V. Bavykin, A. A. Lapkin, P. K. Plucinski, J. M. Friedrich and F. C. Walsh, J. Phys. Chem. B, 2005, 109, 19422–19427 CrossRef CAS PubMed.
- E. Horváth, P. R. Ribiè, F. Hashemi, L. Forró and A. Magrez, J. Mater. Chem., 2012, 22, 8778–8784 RSC.
- S. N. Britvin, A. Lotnyk, L. Kienle, S. V. Krivovichev and W. Depmeier, J. Am. Chem. Soc., 2011, 133, 9516–9525 CrossRef CAS PubMed.
- Y. Tang, Y. Zhang, J. Deng, D. Qi, W. R. Leow, J. Wei, S. Yin, Z. Dong, R. Yazami, Z. Chen and X. Chen, Angew. Chem., Int. Ed., 2014, 53, 13488–13492 CrossRef CAS PubMed.
- W. J. Dong, Y. J. Zhu, H. D. Huang, L. S. Jiang, H. J. Zhu, C. R. Li, B. Y. Chen, Z. Shi and G. Wang, J. Mater. Chem. Eng., 2013, 1, 10030–10036 CAS.
- J. Cheng, R. C. Che, C. Y. Liang, J. W. Liu, M. Wang and J. J. Xu, Nano Res., 2014, 7, 1043–1053 CrossRef CAS.
- Z. H. Liu, X. J. Su, G. L. Hou, S. Bi, Z. Xiao and H. P. Jia, Nanoscale, 2013, 5, 8177–8183 RSC.
- J. Wang, T. Qiu, X. Chen, Y. L. Lu and W. S. Yang, J. Power Sources, 2014, 268, 341–348 CrossRef CAS PubMed.
- S. Laurent, D. Forge, M. Port, A. Roch, C. Robic, L. V. Elst and R. N. Muller, Chem. Rev., 2008, 108, 2064–2110 CrossRef CAS PubMed.
- S. H. Xuan, Y. J. Wang, J. C. Yu and K. C. Leung, Chem. Mater., 2009, 21, 5079–5087 CrossRef CAS.
- W. Cheng, K. B. Tang, Y. X. Qi, J. Sheng and Z. P. Liu, J. Mater. Chem., 2010, 20, 1799–1805 RSC.
- J. P. Ge, Y. X. Hu, M. Biasini, W. P. Beyermann and Y. D. Yin, Angew. Chem., Int. Ed., 2007, 46, 4342–4345 CrossRef CAS PubMed.
- S. H. Xuan, W. Q. Jiang, X. L. Gong, Y. Hu and Z. Y. Chen, J. Phys. Chem. C, 2009, 113, 55–558 Search PubMed.
- W. Li, Y. H. Deng, Z. X. Wu, X. F. Qian, J. P. Yang, Y. Wang, D. Gu, F. Zhang, B. Tu and D. Y. Zhao, J. Am. Chem. Soc., 2011, 133, 15830–15833 CrossRef CAS PubMed.
- J. W. Liu, J. J. Xu, R. C. Che, H. J. Chen, M. M. Liu and Z. W. Liu, Chem.–Eur. J., 2013, 19, 6746–6752 CrossRef CAS PubMed.
- J. N. Gao, X. Z. Ran, C. M. Shi, H. M. Cheng, T. M. Cheng and Y. P. Su, Nanoscale, 2013, 5, 7026–7033 RSC.
- W. Stöber, A. Fink and E. Bohn, J. Colloid Interface Sci., 1968, 26, 62–69 CrossRef.
- Y. W. Wang, H. Xu, X. B. Wang, X. Zhang, H. M. Jia, L. Z. Zhang and J. R. Qiu, J. Phys. Chem. B, 2006, 110, 13835–13840 CrossRef CAS PubMed.
- Y. F. Tang, L. Yang, J. Z. Chen and Z. Qiu, Langmuir, 2010, 26, 10111–10114 CrossRef CAS PubMed.
- X. X. Yu, S. W. Liu and J. G. Yu, Appl. Catal., B, 2011, 104, 12–20 CrossRef CAS PubMed.
- R. Z. Ma, K. Fukuda, T. Sasaki, M. Osada and Y. Bando, J. Phys. Chem. B, 2005, 109, 6210–6214 CrossRef CAS PubMed.
- Q. Zhang, T. R. Zhang, J. P. Ge and Y. D. Yin, Nano Lett., 2008, 8, 2867–2871 CrossRef CAS PubMed.
- W. Q. Cai, J. G. Yu and M. Jaroniec, J. Mater. Chem., 2010, 20, 4587–4594 RSC.
- J. B. Fei, Y. Cui, X. H. Yan, W. Qi, Y. Yang, K. W. Wang, Q. He and J. B. Li, Adv. Mater., 2008, 20, 452–456 CrossRef PubMed.
- M. R. Gao, S. R. Zhang, J. Jiang, Y. R. Zheng, D. Q. Tao and S. H. Yu, J. Mater. Chem., 2011, 21, 16888–16892 RSC.
- Y. M. Shao, L. C. Zhou, C. Bao and J. J. Ma, Carbon, 2015, 89, 378–391 CrossRef CAS PubMed.
- W. Wang, Z. Ding, M. H. Cai, H. T. Jian, Z. Q. Zeng and F. Li, Appl. Surf. Sci., 2015, 346, 348–353 CrossRef CAS PubMed.
- P. F. Wang, M. H. Cao, C. Wang, Y. H. Ao, J. Hou and J. Qian, Appl. Surf. Sci., 2014, 290, 116–124 CrossRef CAS PubMed.
- L. M. Cui, X. Y. Guo, Q. Wei, Y. G. Wang, L. Gao, L. G. Yan, T. Yan and B. Du, J. Colloid Interface Sci., 2015, 439, 112–120 CrossRef CAS PubMed.
- M. Feng, W. You, Z. S. Wu, Q. D. Chen and H. B. Zhan, ACS Appl. Mater. Interfaces, 2013, 5, 12654–12662 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Zeta potential of Fe3O4 and Fe3O4@titanate microspheres, EDX results of Fe3O4@SiO2@AT and Fe3O4@titanate, and magnetic properties of Fe3O4@titanate microspheres. See DOI: 10.1039/c5ra06362k |
| This journal is © The Royal Society of Chemistry 2015 |

