Robust dual physically cross-linked hydrogels with unique self-reinforcing behavior and improved dye adsorption capacity†
Wei Cui,
Zi-Jing Zhang,
Hang Li,
Le-Min Zhu,
Huan Liu and
Rong Ran*
College of Polymer Science and Engineering, State Key Laboratory of Polymer Materials Engineering, Sichuan University, Chengdu 610065, People's Republic of China. E-mail: ranrong@scu.edu.cn
First published on 9th June 2015
Abstract
Dual physically cross-linked (DPC) hydrogels were facilely fabricated by introducing hectorite clay LAPONITE® XLG into a hydrophobically associated polyacrylamide (HAPAM) system via one-pot in situ polymerization. The DPC gels exhibited excellent mechanical strength and unique self-reinforcing behavior with the aid of an additional cross-linking effect provided by LAPONITE®. More impressively, the self-reinforcement of DPC gels could easily be achieved through any of the following three methods: self-healing, remolding or stretching, which was unrealizable for the reported hydrophobic association hydrogels. Compared with HAPAM gels, improved cationic dye adsorption capacity appeared in DPC gels owing to the existence of abundant negative charges on the surface of LAPONITE®. Our proposed methodology highlights the possibility of designing a new type of readily self-reinforced physically cross-linked hydrogels, which have great application potential in bioengineering and the treatment of organic dyes.
1. Introduction
Hydrogels, a type of three-dimensional hydrophilic cross-linked polymers, have attracted great scientific interest over the past several decades. It has been reported that hydrogels can be applied in various fields such as biomimetic actuators,1–4 tissue engineering,5 molecular filters6,7 and sensors.8 Polymer chains of hydrogels are usually cross-linked by covalent or strong physical bonds and swollen in aqueous solution. Unfortunately, the application range of most hydrogels prepared by chemical cross-linking is seriously restricted because of their irreversible cross-linking pattern, low mechanical strength and poor stability. Moreover, the addition of cross-linking agent makes the hydrogel system impure, which is not desirable for biological applications. Therefore, it is very necessary to improve the performance of conventional synthetic hydrogels in order to broaden their application range.At present, enormous efforts have been devoted to developing tough hydrogels with favorable comprehensive properties, such as double network hydrogels,9–12 sliding-ring hydrogels,13 hydrophobically associated (HA) hydrogels,14 nanocomposite (NC) hydrogels,15 macromolecular microsphere composite hydrogels,16 and dipole–dipole or hydrogen bonding enhanced hydrogels.17,18 Among them, HA gels are proved to possess self-healing19,20 and reforming21 capacity without the introduction of extra cross-linking agent. The gelation results from the cross-linking effect supplied by hydrophobically associated micelles,22 whose role is similar to that of cross-linking agents in chemical cross-linked hydrogels. However, because of the single cross-linking pattern, HA gels usually exhibit weaker mechanical strength after loaded. In addition, the conventional amide monomers based HA gels have been considered feeble in dye adsorption on account of their non-ionic monomer composition. Another class of hydrogels physically cross-linked by nanoscopic inorganic materials such as clay is generally called NC gels. For these clay nanocomposite hydrogels, clay are uniformly dispersed and acted as 2-dimensional and multifunctional cross-linkers by adsorbing polymer chains through hydrogen bonding and electrostatic interaction to form a 3-dimensional network. However, NC gels of single cross-linked network usually perform poor mechanical strength23,24 and undesirable self-healing efficiency at low clay content.25,26 Consequently these inherent drawbacks of HA and NC gels have largely blocked their practical applications. In contrast to single cross-linked hydrogels, DN gels consist of two independently cross-linked networks demonstrate ultrahigh mechanical strength and remarkable toughness.27 Generally, DN gels exhibit the best mechanical properties when the first network is highly cross-linked and the second only lightly cross-linked.28 Nevertheless, the majority of DN gels are manufactured by a time consuming two-step approach, during the second step hydrogels with the first network are swelling in precursor solutions of the second network, resulting in an unmanageable total water content of DN gels. Extending this point of view, developing a class of hydrogel with similar network structure to DN gels through one step method seems an appealing alternative. Some approaches have been proposed in an endeavor to obtain this type of hydrogel, namely the dual cross-linked (DC) hydrogel. The existing knowledge of DC hydrogels mainly comes from hybrid physically–chemically cross-linked hydrogels, whose networks are synchronously cross-linked by covalent bonds and physical interactions.29,30 On account of the irreversible and permanent cross-linking pattern originated from covalent bonds, the self-healing property of hybrid DC hydrogel is usually suppressed seriously, which, however, proves to be a fantastic feature in applications.31,32 For HA and NC gels, though self-healing ability is endowed by reversible cross-linking patterns, the mechanical strength of them after repaired always decreases heavily due to low healing efficiency, resulting in their poor reusability. Reasonably, combining the hydrogel system of HA and NC gels to simultaneously ameliorate the mechanical strength and healing efficiency should be an imperative and instructive attempt.
Self-reinforcement is a desirable property for materials requiring recycle use,33 while it is not readily accessible for hydrogels because of their high water content. Tong et al. observed self-reinforcement from polymer–LAPONITE® NC gels by stretching and tearing.34 The orientation of LAPONITE® during elongation was responsible for the self-reinforcement of NC gels. Up to now, no self-reinforcing behavior has been discovered for HA and conventional DC gels, which seriously limited their other applications demanding repeated use such as cartilage, muscle, and adsorbing material for dye wastewater treatment.
In recent years dye contamination has become a serious environmental and social problem due to their environmental toxicity and public health damage. Polymeric hydrogels has been found to be severed as an optimum adsorbing material for dye wastewater treatment in terms of economic feasibility, adsorption-regeneration, simplicity of design, ease of operation and insensitivity to toxic substances.35 It is known that NC hydrogels can absorb and trap ionic dyes, in which LAPONITE® carrying strong negative charges on surface has been demonstrated powerful in dye adsorption because of their high specific surface area, chemical and mechanical stabilities, and a variety of surface and structural properties.36 However, limited recoverability and self-healing property of NC gels restricted their use for this application. Therefore, it naturally arouses our interest to explore a novel derivation of hydrogels, combining high mechanical strength, self-reinforcing behavior, dye adsorption capacity of HA, NC and DC gels.
The existing literatures lead us to consider that it is supposed not difficult to combine the cross-linking networks of HA and NC gels simultaneously to obtain a type of dual physically cross-linked hydrogels since these two hydrogel systems are quite compatible. As far as we know, although a range of HA hydrogels and NC hydrogels have been reported, in the case of influences controlled by LAPONITE® and hydrophobic/hydrophilic monomers on the mechanical properties, there have been little progress in self-reinforcement of NC or HA gels. To the best of our knowledge, no self-reinforced hydrogel has been reported with the combination of HA and NC hydrogel systems so far.
In this work, we report the successful preparation of novel dual physically cross-linked (DPC) gels with unique self-reinforcing behavior and improved dye adsorption capacity by a simple one-pot polymerization method. The self-reinforcement of DPC gels could be achieved through three methods: self-healing, remolding and stretching, which was totally inaccessible for chemically cross-linked or hybrid DC gels. We have explored a strategy to improve the mechanical properties and self-healing efficiency of hydrogels by combining synergistic cross-linking effect provided by hydrophobically associated micelles and LAPONITE® nanosheets. The LAPONITE® added acts as cooperative cross-linking agent and improves the dye adsorption ability. The results show us an efficient way to synthesize self-reinforced dual physically cross-linked hydrogels for practical application as tough materials in tissue scaffolds and wastewater treatment.
2. Experimental
2.1 Materials
Acrylamide (AM) and potassium persulfate (K2S2O8), purchased from Tianjin Bodi Chemical Co., Ltd, were recrystallized from distilled water before use and dried under vacuum at room temperature. Sodium dodecyl benzene sulfonate (SDBS) was provided by Chengdu Kelong Chemical Reagent Factory, stearyl methylacrylate (SMA) was obtained from J&K Chemical Technology Co., Ltd, both were used without further purification. Synthetic hectorite clay of gel-forming grade LAPONITE® XLG (Mg5.34Li0.66Si8O20(OH)4Na0.66) was kindly provided by Huizhi Fine Chemical Co., Ltd, and used after dried at 125 °C for 2 h. The dyes, malachite green (MG), methylene blue (MB), methyl orange (MO), basic fuchsin (BF) and crystal violet (CV) were purchased from Haihong Chemical Reagent Factory. All solutions used in experiments were prepared in deionized water.2.2 Synthesis of DPC gels
DPC gels were synthesized through in situ copolymerization of AM (10 wt%) and SMA (2 mol% relative to AM) in the aqueous suspension of LAPONITE® (varied) with SDBS (3 wt%), initiated by KPS (0.5 wt% relative to the total mass of AM and SMA), all the total mass of the initial reaction solution was fixed at 15 g. For example, the experimental procedure used for DPC0.5 was as follows: 0.075 g of LAPONITE® was first dispersed in deionized water (12.02 g) under stirring for at least 4 h until a homogeneous suspension was achieved. Then, the hydrophilic monomer AM (1.50 g) and the surfactant SDBS (0.45 g) were added into the LAPONITE® suspension under stirring, 0.14 g of hydrophobic monomer SMA was subsequently put in. The mixture was stirred for another 24 h to make a uniform solution. Finally, the aqueous solution of initiator (KPS 0.0082 g in 0.82 g of water) was added to the former solution. The solution was put into an ampoule and then flame-sealed after being bubbled with N2 for 10 min to eliminate oxygen, the polymerization was allowed to proceed at 50 °C for 5 h, and then DPC gels samples were obtained.In this work, DPC gels were designated as DPCn, where n stood for the mass fraction of LAPONITE® (varying from 0.5 to 1.5) relative to the total mass of initial reaction solution. For comparison, HA and NC gels under the same experimental conditions were also prepared. Nomenclature and composition contents of all gels are listed in Table 1.
| Samples | LAPONITE® [g] | H2Oa [g] | AM [g] | SMA [g] | SDBS [g] | KPS [g] | H2Ob [g] |
|---|---|---|---|---|---|---|---|
| a Mass of water used to disperse LAPONITE® and dissolve monomers.b Mass of water used to dissolve initiator KPS. | |||||||
| NC1 | 0.15 | 12.59 | 1.50 | 0 | 0 | 0.0075 | 0.75 |
| NC1.5 | 0.225 | 12.515 | 1.50 | 0 | 0 | 0.0075 | 0.75 |
| HA | 0 | 12.08 | 1.50 | 0.14 | 0.45 | 0.0082 | 0.82 |
| DPC0.5 | 0.075 | 12.005 | 1.50 | 0.14 | 0.45 | 0.0082 | 0.82 |
| DPC1 | 0.15 | 11.93 | 1.50 | 0.14 | 0.45 | 0.0082 | 0.82 |
| DPC1.5 | 0.225 | 11.855 | 1.50 | 0.14 | 0.45 | 0.0082 | 0.82 |
2.3 Characterization
The morphologies of the hydrogels were examined by a Quanta 250 scanning electron microscope (SEM). To prepare the samples, the swollen hydrogels were equilibrated in distilled water at room temperature, and then quickly frozen and freeze-dried. Afterwards, the freeze-dried samples were fractured carefully, gold-coated and subjected to the SEM analysis.2.4 Mechanical testing
The measurements of tensile mechanical and hysteresis properties were performed on synthesized hydrogels of the same size (10.0 mm diameter × 60 mm length) using an Instron universal test instrument (Model 5576, Instron Instruments, U.S.A.) with a 1 kN load cell at 25 °C. The tensile strain induced was taken as the change in length relative to the initial length of the specimen. Initial cross-section area was used for calculating tensile stress and elastic modulus, which was calculated from the increase in load detected between elongation of 100% and 200%. For the measurements of hysteresis properties, the sample length between the jaws was 30 mm and the strain was restricted at 1500%. The crosshead speed for the tensile and retraction was fixed at 100 mm min−1.Rheology measurements of DPC, HA gels were conducted with Bohlin Gemini 200 rheometer using a parallel plate of diameter 20 mm at 25 °C. First, the dynamic strain sweep from 0.1% to 10% was carried out at angular frequency of 1 rad s−1 to determine the linear viscoelasticity region. Then, the frequency sweep was performed over the frequency range of 0.1–100 rad s−1 at a fixed strain of 1%.
2.5 Dye adsorption research
Desired amounts of dried DPC and HA gels were immersed in 50 mL of 10 mg L−1 cationic dye solutions, respectively. The test temperature was 25 °C. When the equilibrium swelling states of hydrogels were achieved, the dye solutions were extracted from the adsorption system for the analysis of dye concentrations by using a UV/vis spectrophotometer (UV-3600) at the maximum absorption wavelength of each dye. The amounts of dye adsorbed on the hydrogel at equilibrium states, Qe (mg g−1), were defined according to eqn (1):| Qe = (C0 − Ce) × V/m | (1) |
3. Results and discussion
3.1 Network structure and mechanical properties of DPC gels
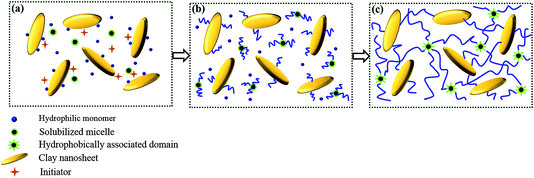
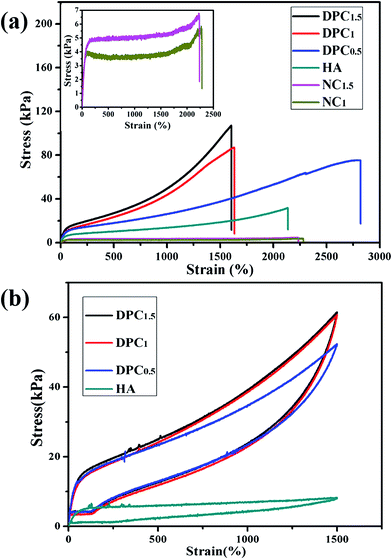
In this study, a series of DPC gels containing varying amounts of LAPONITE® (0, 0.5, 1.0 and 1.5 wt%) were prepared by one-pot in situ polymerization in the LAPONITE® suspension. Compared with conventional chemical hydrogels, no extra cross-linking agent was introduced into the hydrogel system. During the churning process, the hydrophobic monomer SMA was solubilized by SDBS owing to the strong hydrophobic interaction between their hydrophobic side chains, thereby abundant solubilized micelles were formed and stably dispersed in solution37,38 (Scheme 1a), which cross-linked a portion of PAM chains in the process of polymerization to form partial cross-linking junctions.21,39 In the meantime, a number of AM molecules were adsorbed onto LAPONITE® due to their intense interaction (Scheme 1a). Hence some PAM chains propagated from the surfaces of LAPONITE®, resulting in the appearance of grafted chains, one chain end of which was immobilized on the surfaces of LAPONITE® (Scheme 1b). As the grafted chains continued to grow until their active sites at the chain ends encountered others on LAPONITE®, bimolecular terminations happened and integrated PAM chains generated among each LAPONITE® nanosheet40 (Scheme 1c). Thus the LAPONITE® sheets were perceived as another part of cross-linking points. Therefore, dual physically cross-linked networks were formed by the combination of these two cross-linking effects, and bulk hydrogel with three-dimensional polymer networks was constructed.We compared tensile mechanical properties among HA, NC gels and DPC gels. As shown in Fig. 1a, HA gels with single cross-linking network was very ductile and weak (tensile stress of 31.96 kPa). For NC gels with low content of LAPONITE® less than 1 wt%, single cross-linking network could not even support their own weight. When it came to 1 wt% (NC1) or 1.5 wt% (NC1.5), though bulk hydrogels were obtained, their mechanical strength was no more than 7 kPa (inset in Fig. 1a), which was merely 1/18 that of DPC1.5 gel. Therefore, NC gels were eliminated to test for comparison in subsequent measurements since they were too weak to even bear the test conditions. However, when LAPONITE® is introduced into the HA network to form DPC gels, additional cross-linking effect tends to enhance mechanical extensibility, strength, and toughness of the gels. It could also be seen in Fig. 1a that DPC gels exhibited outstanding mechanical properties compared to either HA or NC gel, and when the concentration of LAPONITE® increased from 0.5 to 1.5 wt%, the tensile strength of DPC gels increased obviously. For example, the tensile stress of DPC1 was 87.19 kPa, which was more than twice the total tensile stress of HA and NC1 gel. Therefore, we could conclude from these results that dual cross-linking effects provided by both hydrophobically associated domains and LAPONITE® played the synergistic effect, allowing DPC gels to survive much higher strength than that of single cross-linked hydrogel. The detailed values of tensile parameters were summarized in Table 2.
| Samples | Tensile strength (kPa) | Elongation (%) | Tensile modulus (kPa) | The maximum load (N) |
|---|---|---|---|---|
| NC1 | 5.71 | 2270 | 0.12 | 0.25 |
| NC1.5 | 6.96 | 2235 | 0.44 | 0.39 |
| HA | 31.96 | 2136 | 0.99 | 2.19 |
| DPC0.5 | 75.17 | 2821 | 1.81 | 5.32 |
| DPC1 | 87.19 | 1633 | 2.91 | 6.33 |
| DPC1.5 | 107.11 | 1605 | 3.06 | 6.96 |
In Fig. 1b we compared the tensile hysteresis curves of DPC and HA gels during a loading–unloading cycle to reveal their energy dissipation capacity, which was another indicator to assess the mechanical property of hydrogels. Hysteresis loops were observed when DPC and HA gels were stretched to 1500% and reverted at the same speed of 100 mm min−1. At strain of 1500%, all DPC gels exhibited similar large hysteresis loops, which extended a little with increasing LAPONITE® content. While single cross-linked HA gel only showed a very small hysteresis loop. Consistently, DPC0.5, DPC1 and DPC1.5 gels had almost equivalent dissipated energies of 147.63, 188.43 and 188.62 kJ m−3, respectively, which was much higher than that of 43.10 kJ m−3 for HA gel. One could conclude that energy was dissipated much more efficiently by DPC gels than HA gel during the stretching and reverting, thus resulting in higher mechanical strength and toughness.
3.2 Rheology measurements
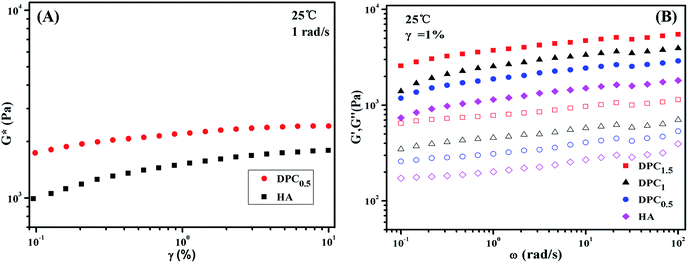
The shear strain γ dependence of the complex modulus G* was tested at 25 °C and 1 rad s−1 for HA and DPC0.5 to determine the linear viscoelasticity region of the as-prepared hydrogels. It can be seen from Fig. 2A that the absolute value of G* turns independent of γ over the range from 1% to 10%. Hence all the viscoelasticity measurements were conducted at γ = 1% to ensure the availability of the linear viscoelasticity and enough sensitivity.In order to enhance the stability of the pre-polymerization suspension and increase the mechanical properties of conventional single cross-linked HA gels, a reinforcing agent compatible with the polymer in water was required. LAPONITE® was applicable not only for the stabilization of the pre-polymerization suspension but also for the enhancement to the hydrogels, which played the role of cross-linking agent. Fig. 2B illustrates the angular frequency ω dependence of the storage modulus G′ and loss modulus G′′ for HA and DPC gels with different LAPONITE® content. It is obvious that G′ is always much higher than G′′ and appeared as a plateau over the observed frequency range for HA and all DPC gels, which indicates that cross-linked networks have been formed in these gels. It is noted that addition of only 0.5 wt% of LAPONITE® leads to an evident increase in G′ compared to HA gels. Moreover, higher LAPONITE® concentration gives rise to higher G′, which is consistent with the previous tensile tests of the hydrogels. At the frequency of 1 rad s−1, the G′ of DPC0.5 is 1872 Pa, higher than the 1143 Pa of HA, when the amount of LAPONITE® increased to 1.5 wt%, the G′ reached 3726 Pa, which is 226% higher than that of HA gel. These results suggest the excellent physically cross-linking effect provided by LAPONITE® appears in DPC hydrogels.
3.3 Effective network chain density
To further understand the enhancement mechanism of DPC gels, we determined the network chain density from the equilibrium shear modulus based on the rubber elasticity at a small strain of 1% to ensure availability of the assumptions in the theory. The effective network chain density N was calculated according to the formula as in ref. 41 and 42.| Ge = NRT | (2) |
For comparison, the network chain density was also estimated by using the elongation date following the method reported in the literature.43,44 For elongation, the effective network chain density N* is evaluated from the tensile stress τ and elongation ratio γ on the assumption of affine deformation and incompressible volume.
| τ = N*RT[γ − (1/γ)2] | (3) |
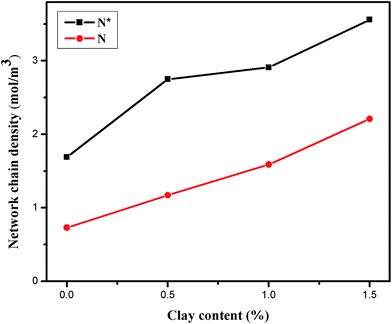 | ||
| Fig. 3 The effective network chain density of DPC gels (HA gel was expressed as DPC0) with varying LAPONITE® content. | ||
3.4 Morphological analysis
The internal microstructures of HA gel and DPC1.5 gel were characterized by SEM. As shown in Fig. 4, it could be seen that the HA gel presented a heterogeneous network structure with plenty of wrinkles (Fig. 4a1), while DPC1.5 showed a much denser homogeneous structure with well-defined pores (Fig. 4b1). Notably, the introduction of LAPONITE® showed a dramatic influence on the wall of the pores in the hydrogels. The existence of LAPONITE® gave rise to a significant increase in the wall thickness of DPC1.5 (Fig. 4a2) in clear comparison to that of HA gel (Fig. 4b2), which indicated that a more compact network structure was generated in DPC1.5 gel by the introduction of LAPONITE®. Hence when loaded, more effective energy dissipation could be achieved by DPC1.5 network, resulting in an improvement of mechanical properties, which was consistent with Fig. 1. Moreover, it was easier for dye molecules to be absorbed into DPC gels due to the capillarity caused by smaller and more uniform pores.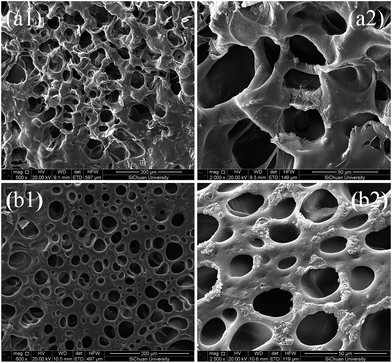 | ||
| Fig. 4 SEM micrographs of (a1) HA gel, (a2) a local partial enlarged image of (a1), (b1) DPC1.5 gel, (b2) a local partial enlarged image of (b1). | ||
3.5 Self-reinforcement of DPC gels achieved by self-healing, remolding and stretching
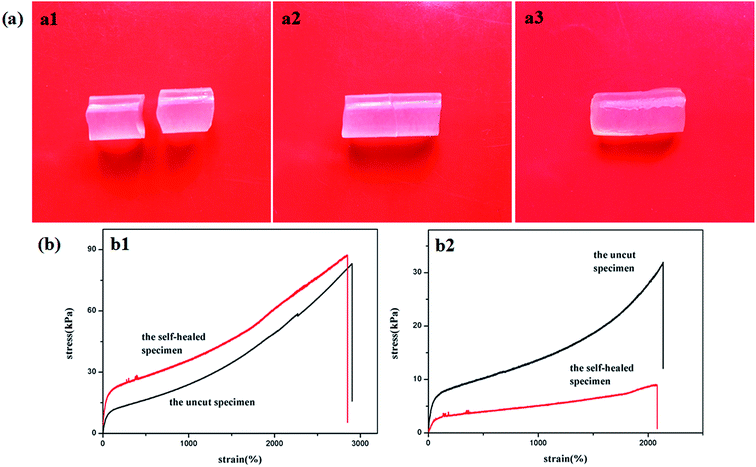
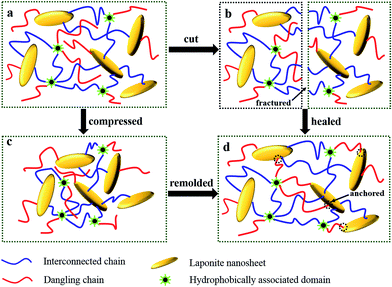
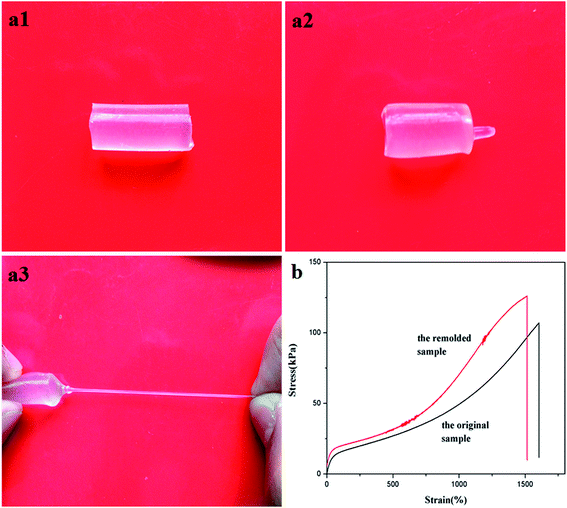
Dual physically cross-linked network was created in DPC gels by the introduction of LAPONITE® without affecting another cross-linking effect, which endowed DPC gels excellent properties such as self-reinforcement and dye adsorption capacity, meanwhile, some excellent properties of HA gels such as self-healing and reforming, were still kept in DPC gels.Self-healing ability of DPC gels was inherited from HA and NC gels. The self-healing process of DPC0.5 gel was shown in Fig. 5a. The hydrogel specimen was cut into two separate parts (Fig. 5a1), then the two pieces were connected immediately after a small amount of water was sprayed on the cut surfaces (Fig. 5a2). The self-healed DPC0.5 gel without any sign of a knife-cut was obtained by preserving the cut pieces at room temperature in wet-keeping cabinet for 3 days (Fig. 5a3). We further evaluated the self-healing efficiency of DPC0.5 gel by tensile measurements, which of HA gel was also investigated as a comparison. As depicted in Fig. 5b, both of the self-healed HA gel and DPC0.5 gel still possessed high extensibility and could be stretched to a large deformation without breaking. Interestingly, it was noted that the tensile modulus, strength of the self-healed DPC0.5 gel exceeded the original specimen (Fig. 5b1) in contrast with the sharp decrease in those of HA gel after self-healing (Fig. 5b2), suggesting the self-healing efficiency of DPC0.5 was superior to that of HA gel apparently and self-reinforcement of DPC gels could be attained by means of self-healing.
It was reported that the conventional HA gels were endowed self-healing ability due to the reversible dissociation process of the physical cross-linking inside the hydrogel network.46,47 Unfortunately, the absence of long and flexible polymer chain entanglements after cut usually led to a significant decrease in mechanical strength even after the HA specimen was healed. Thus self-reinforcement was totally unrealizable for HA gels without the aid of cross-linking effect supplied by LAPONITE®. The unique polymer–clay dual cross-linked network structure formed in DPC gels by the introduction of LAPONITE® played an essential role in improving the strength of DPC gels after healed. Once the DPC gel was obtained after polymerization, numerous dangling polymer chains with only one end anchored to LAPONITE® nanosheets and hydrophobically associated domains appeared in the hydrogel network (redlines in Scheme 2a), which contributed nothing to the mechanical properties of DPC gels. But when the two cut pieces (Scheme 2b) were connected, the mobility of polymer chains at the fresh-cut surfaces was enhanced owning to the lubrication of water, thus most of these dangling chains could be adsorbed onto the adjacent LAPONITE® nanosheets through chain diffusion and hydrogen bonding,48,49 which strengthened the cross-linking effect at the joint section. Meanwhile, additional cross-linking effect supplied by hydrophobic association domains was created again due to the structural reorganization of hydrophobically modified PAM chains.50,51 As a consequence, intensive cross-linked network was formed at the self-healing interface by the synergistic effect of dual recross-linking actions (Scheme 2d), resulting in a mechanical enhancement of DPC gels. That is, more hydrogen interactions between polymer chains and LAPONITE® nanosheets would appear in DPC gels after healed, which would need more energy for the breaking of the hydrogels. Thereby the tensile toughness of DPC0.5 after healed was also increased in obviously contrast with HA since the tensile toughness was defined as the area underneath the tensile stress–strain curve (Fig. S1†).
In addition to self-healing ability, the structural reorganization of hydrophobically modified PAM chains also endowed DPC gels with remolding capacity as well as HA gels.39 As shown in Fig. 6a, the forepart of the original DPC1.5 (Fig. 6a1) entirely copied the shape of a syringe nozzle after being forced into the syringe with pressing the plunger for 3 h at 50 °C (Fig. 6a2). The remolded part could still be stretched to a large deformation before breaking (Fig. 6a3). Tensile measurements were conducted to evaluate the mechanical strength of DPC1.5 after remolding (Fig. 6b). It was remarkable that the self-reinforcement emerged in the remolded part by comparison of tensile toughness, which was consistent with the self-healing results (Fig. S2†).
We suspected that the dangling chains on LAPONITE® played a crucial part again in enhancing DPC gels after remolding. When DPC1.5 was compressed in syringe, the distance between neighboring LAPONITE® nanosheets was shortened (Scheme 2c). Moreover, the motility of polymer chains was enhanced thanks to the elevated temperature, offering more opportunities for dangling chains to adhere onto the surfaces of LAPONITE® nanosheets. The cross-linking density would inevitably increase as long as the remolding process period was long enough (Scheme 2d), resulting a reinforcement in mechanical strength of DPC gels. Both hydrophobically associated domains and LAPONITE® nanosheets performed its own cross-linking effect in remolding process. Reversible cross-linking action of hydrophobically associated domains allowed DPC gels to reform into other shapes, at the same time the adsorption of dangling chains onto LAPONITE® contributed to the increase in mechanical strength of DPC gels after remolding. Therefore, not only could DPC gels be shape-molded in polymerization process, but also would be remolded by reforming process as thermoplastic resins to obtain stronger mechanical properties, which was totally impossible for hybrid dual cross-linked hydrogels because of the irreversible covalent cross-linked bonds.52 It was worth nothing that the self-reinforcement process achieved by self-healing and remolding could not be repeated unlimitedly owning to the limited number of dangling chain, which would be discussed in our further work.
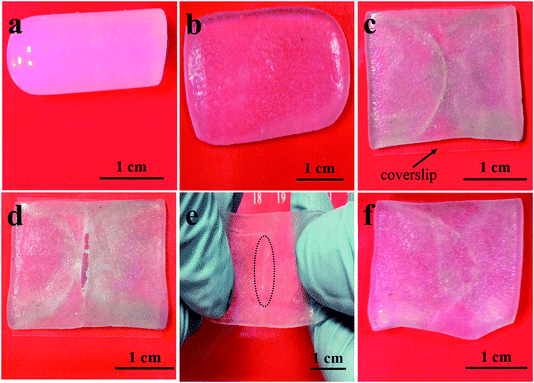
Intriguingly, by combining the self-healing and remolding abilities, DPC gels could be employed as a sort of self-healing protecting film materials. In this scenario, we were triggered to verify the protective action of DPC gels on easily scratched materials. DPC1.5 gel was demonstrated as an example (Fig. 7a). By forcibly compressing DPC1.5 for 3 h, it completely remolded to be a thin film (Fig. 7b). Then we coated a coverslip with DPC1.5 as a protecting film (Fig. 7c). To imitate the scratch on materials in practical application, a wound was created through knife-cutting (Fig. 7d), which, however, did not appear on the surface of coverslip thanks to the protection of DPC1.5 film. After closing the notch together and spraying trace amount of water on the cut, the hydrogel film self-healed at room temperature after 3 days, which is found to be inseparable even if the film was stretched (Fig. 7e). The self-healed gel could be utilized again as protecting film on the coverslip (Fig. 7f). The results obtained here were expected to help widen the practical application of DPC gels due to their fantastic properties by combination of HA and NC hydrogel systems.
Stress softening was discovered in HA gels,39 namely, tensile force of HA gels tended to decrease for consecutive tests since some hydrophobic association micelles was broken in tensile process. However, polymer–LAPONITE® NC gels usually showed an enhanced mechanical behavior after stretching to a large deformation due to the orientation of LAPONITE® nanosheets in the NC gel during the elongation.15,48,53 The contradictory repeat tensile property between HA gels and NC gels naturally arouses our interest to investigate the repeat tensile property of DPC gels, whose system was a combination of HA and NC gels. Fig. 8 showed the stress–strain curves of DPC gels during the continuous elongations at a fixed strain of 1200% for five times. The stress increased with strain obviously during subsequent tensile after the first stretching. And no fracture appeared during the elongation, suggesting the outstanding tensibility and self-reinforcement property of DPC gels. Thus it could be concluded that the orientation of LAPONITE® nanosheets contributed a lot to enhance the mechanical strength and neutralize the reduction caused by the damage of hydrophobically associated micelles.
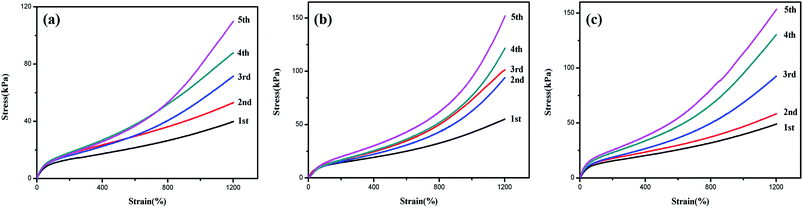 | ||
| Fig. 8 Stress–strain curves for DPC0.5 (a), DPC1 (b) and DPC1.5 (c) gel under repeated stretching to a fixed strain at 1200% for five times. | ||
To sum up of this section, self-reinforcement of DPC gels can be realized by three methods: self-healing, remolding and repeated stretching to a constant strain, which may widen the application of hydrogels in the field of materials requiring recycling use.
3.6 Improved dye adsorption capacity of DPC gels
As is known to all, hydrogels often have porous network structure and allow solute diffusion through the hydrogel structure.54 After introducing LAPONITE® bearing abundant negative charges into HA gels, the cationic dye adsorption capacity should be further enhanced.A series of water soluble cationic dyes (MG, MB, MO, BF and CV) were used for studying the dye adsorption capacity of DPC gels (DPC1.5 was shown as an example in Fig. 9), the dye adsorption performance of DPC gels can be clearly observed by the change in depth of the dye colors. After immersing in each dye solution (10 mg L−1) (Fig. 9a) for 6 h at room temperature, DPC1.5 turned its color into the same color of the dye while the dye solution faded obviously (Fig. 9b). Then we took all the samples out and immersed them in excess deionized water for 6 h, no leakage was observed and the water always kept clear, indicating a perdurable dye adsorption capacity of DPC gels.
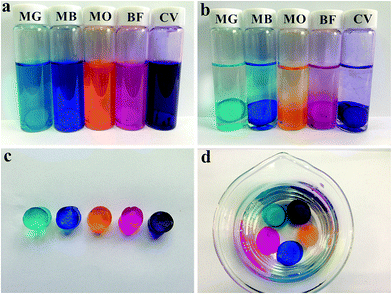 | ||
| Fig. 9 Photographs of dye solutions (a) before and (b) after adsorption, (c) DPC gels after adsorption of dyes, and (d) DPC gels after immersed in excess deionized water for 3 h. | ||
To provide further insight and quantificational evidence for the improved dye adsorption capacity of DPC gels, equilibrium dye adsorption capacities of HA and DPC gels were investigated. The results are listed in Table 3. It can be seen from that HA gels have certain abilities to adsorb cationic dyes because of their high swelling ratio, whereas DPC gels possess preferable adsorption capacities compared with HA gels. To compare the dye adsorption capacity more quantificationally, time-dependent adsorption of CV on DPC1.5 and HA was conducted as an example (Fig. S3a†). Apparently, the adsorption rate of CV on DPC1.5 was much higher than that on HA, which was reflected by the higher initial slope of the adsorption kinetic curve. Impressively, the equilibrium adsorption amount of CV on DPC1.5 outperformed that on HA with an increase of about 740%, indicating that strong driving force for the adsorption of dyes was generated by the introduction of LAPONITE®. As for the pseudo-second-order equation, the correlation coefficients of CV on DPC1.5 and HA were 0.997 and 0.981, respectively (Fig. S3b†), suggesting that the adsorption kinetics was well in line with the pseudo-second-order model, which was usually used to forecast the amount of dye adsorbed on hydrogel at a certain time. Since there are a lot of negative charges existed on the surfaces of LAPONITE® nanosheets, which are able to interact with cationic dye molecules. Therefore, although the network of DPC gels is more compact, they tend to adsorb more cationic dye molecules instead of water molecules during the swelling process. By this way, DPC gels are endowed superior capacity to adsorb high amount of cationic dye molecules. Thus, with the introduction of LAPONITE®, the mechanical tough DPC gels can be widely used in the treatment of dye pollution.
| Samples | Qe (mg g−1)/MG | Qe (mg g−1)/MB | Qe (mg g−1)/MO | Qe (mg g−1)/BF | Qe (mg g−1)/CV |
|---|---|---|---|---|---|
| HA | 19.912 | 17.398 | 14.265 | 36.868 | 11.737 |
| DPC0.5 | 37.681 | 45.482 | 39.362 | 41.824 | 31.219 |
| DPC1 | 61.273 | 49.420 | 42.529 | 44.221 | 57.851 |
| DPC1.5 | 68.158 | 53.368 | 49.875 | 66.053 | 95.736 |
4. Conclusions
In conclusion, hydrophobically associated PAM hydrogels were introduced with hectorite clay nanosheets LAPONITE® XLG to enhance the mechanical performance and dye adsorption capacity. A series of dual physically cross-linked hydrogels (DPC) were successfully prepared by in situ copolymerization. The LAPONITE® nanosheets acted as fresh physical cross-linking points in hydrogel formation, leading to a significant increase in mechanical properties. Meantime, synergistic cross-linking effect appeared due to the unexceptionable compatibility between LAPONITE® and the hydrophobically associated system, giving rise to an effortlessly achieved self-reinforcement of DPC gels. The self-reinforcement of DPC gels could be accessed by self-healing, remolding and stretching, which was unrealizable for HA and hybrid dual cross-linked gels. Furthermore, DPC gels exhibited much improved cationic dye adsorption capacities compared with HA gels since large amount of negative charges existed on the surfaces of LAPONITE® nanosheets. The novel mechanical strong DPC gels could be expected to widen the applications of HA gels in wastewater treatment and bioengineering.Acknowledgements
This work was supported by Sichuan Ministry of Science and Technology Innovation Talent Project (2013RZX0011), National Natural Science Foundation of China (no. 51173121) and Sichuan Ministry of Science, Technology Project (2013GZX0158), the Innovation Team Program of Science & Technology Department of Sichuan Province (Grant 2014TD0002), and the Applied Basic Research Project of Sichuan Province (Grant 2014JY0074).References
- D. Sivakumaran, D. Maitland, T. Oszustowicz and T. Hoare, Tuning drug release from smart microgel–hydrogel composites via cross-linking, J. Colloid Interface Sci., 2013, 392, 422–430 CrossRef CAS PubMed.
- L. Dong, A. K. Agarwal, D. J. Beebe and H. Jiang, Adaptive liquid microlenses activated by stimuli-responsive hydrogels, Nature, 2006, 442, 551–554 CrossRef CAS PubMed.
- L. Ionov, Biomimetic Hydrogel-Based Actuating Systems, Adv. Funct. Mater., 2013, 23, 4555–4570 CrossRef CAS PubMed.
- Z. L. Wu, M. Moshe, J. Greener, H. Therien-Aubin, Z. Nie, E. Sharon and E. Kumacheva, Three-dimensional shape transformations of hydrogel sheets induced by small-scale modulation of internal stresses, Nat. Commun., 2013, 4, 1586–1592 CrossRef PubMed.
- K. Crompton, J. Goud, R. Bellamkonda, T. Gengenbach, D. Finkelstein, M. Horne and J. Forsythe, Polylysine-functionalised thermoresponsive chitosan hydrogel for neural tissue engineering, Biomaterials, 2007, 28, 441–449 CrossRef CAS PubMed.
- G. Rama Rao, M. E. Krug, S. Balamurugan, H. Xu, Q. Xu and G. P. López, Synthesis and characterization of silica–poly(N-isopropylacrylamide) hybrid membranes: switchable molecular filters, Chem. Mater., 2002, 14, 5075–5080 CrossRef.
- M. Guilherme, R. Silva, E. Girotto, A. Rubira and E. Muniz, Hydrogels based on PAAm network with PNIPAAm included: hydrophilic–hydrophobic transition measured by the partition of Orange II and Methylene Blue in water, Polymer, 2003, 44, 4213–4219 CrossRef CAS.
- Y. J. Lee and P. V. Braun, Tunable inverse opal hydrogel pH sensors, Adv. Mater., 2003, 15, 563–566 CrossRef CAS PubMed.
- J.-Y. Sun, X. Zhao, W. R. Illeperuma, O. Chaudhuri, K. H. Oh, D. J. Mooney, J. J. Vlassak and Z. Suo, Highly stretchable and tough hydrogels, Nature, 2012, 489, 133–136 CrossRef CAS PubMed.
- J. P. Gong, Y. Katsuyama, T. Kurokawa and Y. Osada, Double-Network Hydrogels with Extremely High Mechanical Strength, Adv. Mater., 2003, 15, 1155–1158 CrossRef CAS PubMed.
- M. A. Haque, T. Kurokawa, G. Kamita and J. P. Gong, Lamellar bilayers as reversible sacrificial bonds to toughen hydrogel: hysteresis, self-recovery, fatigue resistance, and crack blunting, Macromolecules, 2011, 44, 8916–8924 CrossRef CAS.
- Q. Chen, L. Zhu, C. Zhao, Q. Wang and J. Zheng, A Robust, One-Pot Synthesis of Highly Mechanical and Recoverable Double Network Hydrogels Using Thermoreversible Sol–Gel Polysaccharide, Adv. Mater., 2013, 25, 4171–4176 CrossRef CAS PubMed.
- S. Tan, A. Blencowe, K. Ladewig and G. G. Qiao, A novel one-pot approach towards dynamically cross-linked hydrogels, Soft Matter, 2013, 9, 5239–5250 RSC.
- M. Yang, C. Liu, Z. Li, G. Gao and F. Liu, Temperature-responsive properties of poly(acrylic acid-co-acrylamide) hydrophobic association hydrogels with high mechanical strength, Macromolecules, 2010, 43, 10645–10651 CrossRef CAS.
- K. Haraguchi and H.-J. Li, Mechanical properties and structure of polymer–clay nanocomposite gels with high clay content, Macromolecules, 2006, 39, 1898–1905 CrossRef CAS.
- J. Zhao, K. Jiao, J. Yang, C. He and H. Wang, Mechanically strong and thermosensitive macromolecular microsphere composite poly(N-isopropylacrylamide) hydrogels, Polymer, 2013, 54, 1596–1602 CrossRef CAS PubMed.
- T. Bai, P. Zhang, Y. Han, Y. Liu, W. Liu, X. Zhao and W. Lu, Construction of an ultrahigh strength hydrogel with excellent fatigue resistance based on strong dipole–dipole interaction, Soft Matter, 2011, 7, 2825–2831 RSC.
- L. Tang, W. Liu and G. Liu, High-Strength Hydrogels with Integrated Functions of H-bonding and Thermoresponsive Surface-Mediated Reverse Transfection and Cell Detachment, Adv. Mater., 2010, 22, 2652–2656 CrossRef CAS PubMed.
- D. C. Tuncaboylu, M. Sari, W. Oppermann and O. Okay, Tough and self-healing hydrogels formed via hydrophobic interactions, Macromolecules, 2011, 44, 4997–5005 CrossRef CAS.
- U. Gulyuz and O. Okay, Self-healing polyacrylic acid hydrogels, Soft Matter, 2013, 9, 10287–10293 RSC.
- G. Jiang, C. Liu, X. Liu, Q. Chen, G. Zhang, M. Yang and F. Liu, Network structure and compositional effects on tensile mechanical properties of hydrophobic association hydrogels with high mechanical strength, Polymer, 2010, 51, 1507–1515 CrossRef CAS PubMed.
- C. Liu, J. Yu, G. Jiang, X. Liu, Z. Li, G. Gao and F. Liu, Thermosensitive poly(N-isopropylacrylamide) hydrophobic associated hydrogels: optical, swelling/deswelling, and mechanical properties, J. Mater. Sci., 2013, 48, 774–784 CrossRef CAS.
- K. Haraguchi and T. Takehisa, Nanocomposite hydrogels: a unique organic–inorganic network structure with extraordinary mechanical, optical, and swelling/de-swelling properties, Adv. Mater., 2002, 14, 1120 CrossRef CAS.
- K. Haraguchi, M. Ebato and T. Takehisa, Polymer–clay nanocomposites exhibiting abnormal necking phenomena accompanied by extremely large reversible elongations and excellent transparency, Adv. Mater., 2006, 18, 2250–2254 CrossRef CAS PubMed.
- K. Haraguchi, K. Uyama and H. Tanimoto, Self-healing in Nanocomposite Hydrogels, Macromol. Rapid Commun., 2011, 32, 1253–1258 CrossRef CAS PubMed.
- L. Xiong, X. Hu, X. Liu and Z. Tong, Network chain density and relaxation of in situ synthesized polyacrylamide/hectorite clay nanocomposite hydrogels with ultrahigh tensibility, Polymer, 2008, 49, 5064–5071 CrossRef CAS PubMed.
- J. P. Gong, Why are double network hydrogels so tough?, Soft Matter, 2010, 6, 2583–2590 RSC.
- T. C. Suekama, J. Hu, T. Kurokawa, J. P. Gong and S. H. Gehrke, Double-network strategy improves fracture properties of chondroitin sulfate networks, ACS Macro Lett., 2013, 2, 137–140 CrossRef CAS.
- S. A. Robb, B. H. Lee, R. McLemore and B. L. Vernon, Simultaneously physically and chemically gelling polymer system utilizing a poly (NIPAAm-co-cysteamine)-based copolymer, Biomacromolecules, 2007, 8, 2294–2300 CrossRef CAS PubMed.
- D. C. Tuncaboylu, A. Argun, M. P. Algi and O. Okay, Autonomic self-healing in covalently crosslinked hydrogels containing hydrophobic domains, Polymer, 2013, 54, 6381–6388 CrossRef CAS PubMed.
- B. Yang, Y. Zhang, X. Zhang, L. Tao, S. Li and Y. Wei, Facilely prepared inexpensive and biocompatible self-healing hydrogel: a new injectable cell therapy carrier, Polym. Chem., 2012, 3, 3235–3238 RSC.
- J. Cui and A. del Campo, Multivalent H-bonds for self-healing hydrogels, Chem. Commun., 2012, 48, 9302–9304 RSC.
- C. Gao, L. Yu, H. Liu and L. Chen, Development of self-reinforced polymer composites, Prog. Polym. Sci., 2012, 37, 767–780 CrossRef CAS PubMed.
- C. Lian, Z. Lin, T. Wang, W. Sun, X. Liu and Z. Tong, Self-Reinforcement of PNIPAm–LAPONITE® Nanocomposite Gels Investigated by Atom Force Microscopy Nanoindentation, Macromolecules, 2012, 45, 7220–7227 CrossRef CAS.
- Q. Zhang, T. Zhang, T. He and L. Chen, Removal of crystal violet by clay/PNIPAm nanocomposite hydrogels with various clay contents, Appl. Clay Sci., 2014, 90, 1–5 CrossRef CAS PubMed.
- P. Liu and L. Zhang, Adsorption of dyes from aqueous solutions or suspensions with clay nano-adsorbents, Sep. Purif. Technol., 2007, 58, 32–39 CrossRef CAS PubMed.
- S. Biggs, J. Selb and F. Candau, Effect of surfactant on the solution properties of hydrophobically modified polyacrylamide, Langmuir, 1992, 8, 838–847 CrossRef CAS.
- A. J. Dualeh and C. A. Steiner, Hydrophobic microphase formation in surfactant solutions containing an amphiphilic graft copolymer, Macromolecules, 1990, 23, 251–255 CrossRef CAS.
- G. Jiang, C. Liu, X. Liu, G. Zhang, M. Yang and F. Liu, Construction and properties of hydrophobic association hydrogels with high mechanical strength and reforming capability, Macromol. Mater. Eng., 2009, 294, 815–820 CrossRef CAS PubMed.
- K. Haraguchi, H.-J. Li, K. Matsuda, T. Takehisa and E. Elliott, Mechanism of forming organic/inorganic network structures during in situ free-radical polymerization in PNIPA–clay nanocomposite hydrogels, Macromolecules, 2005, 38, 3482–3490 CrossRef CAS.
- J. Nie, B. Du and W. Oppermann, Swelling, elasticity, and spatial inhomogeneity of poly(N-isopropylacrylamide)/clay nanocomposite hydrogels, Macromolecules, 2005, 38, 5729–5736 CrossRef CAS.
- O. Okay and W. Oppermann, Polyacrylamide–clay nanocomposite hydrogels: rheological and light scattering characterization, Macromolecules, 2007, 40, 3378–3387 CrossRef CAS.
- K. Haraguchi, T. Takehisa and S. Fan, Effects of clay content on the properties of nanocomposite hydrogels composed of poly(N-isopropylacrylamide) and clay, Macromolecules, 2002, 35, 10162–10171 CrossRef CAS.
- K. Haraguchi, R. Farnworth, A. Ohbayashi and T. Takehisa, Compositional effects on mechanical properties of nanocomposite hydrogels composed of poly(N,N-dimethylacrylamide) and clay, Macromolecules, 2003, 36, 5732–5741 CrossRef CAS.
- M. Shibayama, T. Karino, S. Miyazaki, S. Okabe, T. Takehisa and K. Haraguchi, Small-angle neutron scattering study on uniaxially stretched poly(N-isopropylacrylamide)–clay nanocomposite gels, Macromolecules, 2005, 38, 10772–10781 CrossRef CAS.
- X. Hao, H. Liu, Z. Lu, Y. Xie and H. Yang, Endowing the conventional HMPAM hydrogel with pH-responsive and self-healing properties, J. Mater. Chem. A, 2013, 1, 6920–6927 CAS.
- G. Jiang, C. Liu, X. Liu, G. Zhang, M. Yang, Q. Chen and F. Liu, Self-healing Mechanism and Mechanical Behavior of Hydrophobic Association Hydrogels with High Mechanical Strength, J. Macromol. Sci., Pure Appl. Chem., 2010, 47, 335–342 CrossRef CAS PubMed.
- T. Wang, D. Liu, C. Lian, S. Zheng, X. Liu and Z. Tong, Large deformation behavior and effective network chain density of swollen poly(N-isopropylacrylamide)–LAPONITE® nanocomposite hydrogels, Soft Matter, 2012, 8, 774–783 RSC.
- K. Haraguchi, Y. Xu and G. Li, Molecular Characteristics of Poly(N-isopropylacrylamide) Separated from Nanocomposite Gels by Removal of Clay from the Polymer/Clay Network, Macromol. Rapid Commun., 2010, 31, 718–723 CrossRef CAS PubMed.
- E. Volpert, J. Selb and F. Candau, Influence of the hydrophobe structure on composition, microstructure, and rheology in associating polyacrylamides prepared by micellar copolymerization, Macromolecules, 1996, 29, 1452–1463 CrossRef CAS.
- A. Hill, F. Candau and J. Selb, Properties of hydrophobically associating polyacrylamides: influence of the method of synthesis, Macromolecules, 1993, 26, 4521–4532 CrossRef CAS.
- M. C. Roberts, M. C. Hanson, A. P. Massey, E. A. Karren and P. F. Kiser, Dynamically restructuring hydrogel networks formed with reversible covalent crosslinks, Adv. Mater., 2007, 19, 2503–2507 CrossRef CAS PubMed.
- C. Lian, Z. Lin, T. Wang, W. Sun, X. Liu and Z. Tong, Self-reinforcement of PNIPAm–LAPONITE® nanocomposite gels investigated by atom force microscopy nanoindentation, Macromolecules, 2012, 45, 7220–7227 CrossRef CAS.
- S. Li and X. Liu, Synthesis, characterization and evaluation of semi-IPN hydrogels consisted of poly(methacrylic acid) and guar gum for colon-specific drug delivery, Polym. Adv. Technol., 2008, 19, 371–376 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra06361b |
| This journal is © The Royal Society of Chemistry 2015 |