Effect of oxytetracycline on performance and microbial community of an anoxic–aerobic sequencing batch reactor treating mariculture wastewater
Sen Wanga,
Mengchun Gao*ab,
Zhe Wanga,
Zonglian Shea,
Chunji Jina,
Yangguo Zhaoa,
Liang Guoa and
Qingbo Changb
aKey Lab of Marine Environment and Ecology, Ministry of Education, Ocean University of China, Qingdao 266100, China
bCollege of Environmental Science and Engineering, Ocean University of China, No. 238 Songling Road, Qingdao 266100, Shandong, China. E-mail: mengchungao@outlook.com; Fax: +86 532 66782810; Tel: +86 532 66781061
First published on 5th June 2015
Abstract
The performance and microbial community of an anoxic–aerobic sequencing batch reactor (SBR) treating mariculture wastewater were evaluated at different oxytetracycline (OTC) concentrations. The COD and nitrogen removal efficiencies decreased with the increase in OTC concentration from 0 to 12 mg L−1. No apparent NO3−-N accumulation was found during the whole operational period, whereas NO2−-N accumulation occurred at 12 mg L−1 OTC. The specific oxygen uptake rate (SOUR), specific ammonium oxidation rate (SAOR), specific nitrite oxidation rate (SNOR) and specific nitrate reduction rate (SNRR) decreased with increasing influent OTC concentration. The protein (PN) and polysaccharide (PS) contents in the loosely-bound extracellular polymeric substances (LB-EPS) and tightly bound EPS (TB-EPS) increased with the increase in OTC concentration from 0 to 12 mg L−1, respectively. The appearance of OTC in the influent could obviously affect the functional groups of the PN and PS in the LB-EPS and TB-EPS. X-ray photoelectron spectroscopy (XPS) illustrated that amino, carboxyl and hydroxyl groups in the EPS might be involved in the interaction between the EPS and OTC. The denaturing gradient gel electrophoresis (DGGE) profile indicated that some variations were found in the microbial community of the activated sludge with increasing OTC concentration. Some microorganisms were depleted or weakened with the increase in OTC concentration, whereas others became the predominant microorganisms due to their ability to adapt to the OTC toxicity.
Introduction
Mariculture wastewaters mainly originate from the farms of fish, shellfish, crabs and shrimp, which are rich in suspended solids, nitrogenous compounds (i.e. ammonia, nitrite and nitrate) and organic matter.1 Ammonia and nitrite are toxic to aquatic life, while nitrate is implicated in eutrophication and associated with water quality problems. When mariculture wastewaters are directly discharged into the sea without proper treatment, they can lead to algal booms, oxygen depletion and eutrophication in the surrounding estuarine ecosystem.2,3 Effective management of mariculture wastewater is very critical to ensuring the environmental sustainability of the offshore sea area. After suspended solids in mariculture wastewaters are removed by sedimentation, filtration and foam fractionation, biological treatment systems are used to remove dissolved organic matter and nitrogenous compounds. Biological nitrogen removal from mariculture wastewater involves nitrification of ammonia nitrogen (NH4+-N) to nitrate nitrogen (NO3−-N) followed by denitrificaiton of NO3−-N to nitrogen gas (N2). Some biological treatment technologies,4–7 including biological filters, sequencing batch reactors (SBRs), rotating biological contactors and constructed wetland, have been proven to be very effective to remove organic matter and nitrogenous compounds from mariculture wastewaters.Mariculture is a growing industry in response to the dramatic global population growth and the increasing demand for seafood products. As some infectious diseases are the main causes of the economic losses in mariculture and have become a limiting factor for its development, the use of antibiotics has been essential to prevent the spread of pathogenic bacteria.8,9 More antibiotics are released into mariculture wastewater due to the overuse of antibiotics and the presence of metabolites in the excrement.10 Antibiotic pollution may inhibit the microbial activities of different ecosystems, such as nutrient regeneration, organic matter mineralization and pollutant degradation.11–14 Some studies have reported that individual antibiotics have significant effects on the performance of wastewater biological treatment systems treating pharmaceutical wastewater or municipal sewage.15–19 However, the study of antibiotics in mariculture wastewaters is mainly focused on their effects on the ecological toxicity of aquatic organisms in the surrounding estuarine ecosystems in recent years.8,13,20,21
In addition, extracellular polymeric substances (EPS) secreted by microorganisms in biological wastewater treatment systems are regarded as a crucial way to protect cells against the toxicity of antibiotics.22,23 EPS exhibit a dynamic double-layered structure, which consists of loosely bound EPS (LB-EPS) and tightly bound EPS (TB-EPS). EPS have significant influences on the flocculability, dewatering ability, and stability of activated sludge.24 Therefore, it is essential to evaluate the effect of antibiotics on the LB-EPS and TB-EPS of activated sludge treating mariculture wastewater. As far as we know, little information has found in investigating the potential effect of antibiotics on the performance, microbial activity and microbial community of an anoxic–aerobic SBR treating mariculture wastewater, or the effects of antibiotics on the LB-EPS and TB-EPS of activated sludge.
As oxytetracycline (OTC) is one of the most commonly used antibiotics in mariculture, OTC is chosen as a model compound for antibiotics in our study. The objectives of the present study were (a) to investigate the effect of OTC on the COD and nitrogen removal of an anoxic–aerobic SBR treating mariculture wastewater; (b) to analyze the variation in specific oxygen uptake rate (SOUR), specific ammonium oxidation rate (SAOR), specific nitrite oxidation rate (SNOR) and nitrate reduction rate (SNRR) with increasing OTC concentration; (c) to evaluate the effect of OTC on the protein (PN) and polysaccharide (PN) contents in the LB-EPS and TB-EPS of activated sludge; (d) to analyze the variation of functional groups in the LB-EPS and TB-EPS at different OTC concentrations; and (e) to investigate the microbial community in the anoxic–aerobic SBR at different OTC concentrations.
Materials and methods
Reactor set-up and operation
A lab-scale plexiglass anoxic–aerobic SBR was used in the present study. The SBR had an internal diameter of 14 cm and a total height of 55 cm. A peristaltic pump was used to feed the influent into the reactor. The effluent was drawn at a height of 15 cm from the bottom by a solenoid valve, and the volume exchange rate for every cycle was 70%. The mixed liquor in the SBR at the anoxic stage was mixed with a magnetic stirrer, and air was introduced at the aerobic stage via two air diffusers at the bottom of the reactor. The anoxic–aerobic SBR was operated in a 12 h cycle. One cycle consisted of 0.25 h of influent addition, 7 h of aerobic stage, 3 h of anoxic stage, 1.5 h of settling and 0.25 h of effluent withdrawal. The sludge retention time (SRT) of the SBR was 16 d during the operational period, and the mixed liquor suspended solids (MLSS) were kept at about 4500 mg L−1. The system was operated at room temperature (20–30 °C). The dissolved oxygen (DO) concentration at the aerobic stage was over 2.0 mg L−1, and that at the anoxic stage was below 0.5 mg L−1.Seed sludge and synthetic mariculture wastewater composition
The seed sludge was collected from a parent SBR treating synthetic mariculture wastewater in our laboratory. The initial mixed liquor suspended sludge (MLSS) in the anoxic–aerobic SBR was 4500 mg L−1 in the present study. The composition of the synthetic mariculture wastewater is as follows (mg L−1): glucose, 128; NH4Cl, 15.3; KH2PO4, 5; NaNO2, 2.5; NaNO3, 15; and seawater crystals, 3 × 104 (corresponding to 3% salinity). The main components of the seawater crystal solution at 3% salinity were as follows (mg L−1): Na+, 9880; Cl−, 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 025; Mg2+, 950; SO42−, 2500; K+, 360; Ca2+, 300; Zn2+, 0.015; Mn2+, 0.013; Fe2+, 0.13; Co2+, 3 × 10−4; Mo6+, 3 × 10−3; I−, 0.07; Sr+, 7.5 × 10−3; I−, 70; Se6+, 3.5 × 10−4.
025; Mg2+, 950; SO42−, 2500; K+, 360; Ca2+, 300; Zn2+, 0.015; Mn2+, 0.013; Fe2+, 0.13; Co2+, 3 × 10−4; Mo6+, 3 × 10−3; I−, 0.07; Sr+, 7.5 × 10−3; I−, 70; Se6+, 3.5 × 10−4.
Analytical methods
The chemical oxygen demand (COD), ammonia nitrogen (NH4+-N), nitrite nitrogen (NO2−-N), nitrate nitrogen (NO3−-N), mixed liquor suspended solids (MLSS), sludge volume index (SVI) and mixed liquor volatile suspended solids (MLVSS) were measured according to Chinese NEPA standard methods.25A heat extraction method was used to extract LB-EPS and TB-EPS from activated sludge.26 The PN contents in LB-EPS and TB-EPS were measured according to the Lowry method.27 The PS contents in LB-EPS and TB-EPS were determined using the anthrone–sulfuric acid method.28 Three-dimensional excitation–emission matrix (3D-EEM) fluorescence spectra of LB-EPS and TB-EPS were recorded according to Wang et al.26 The X-ray photoelectron spectroscopy (XPS) of LB-EPS and TB-EPS was analyzed according to Wang et al.29
Determination of SOUR, SAOR, SNOR and SNRR
The SOUR, SAOR, SNOR and SNRR of the activated sludge were measured according to Wang et al.,30 with minor modification, on days 25, 50, 73, 95, 130 and 160, corresponding to the OTC concentrations of 0, 2, 4, 8, 10 and 12 mg L−1, respectively. The SOUR determination was performed in a 500 mL pre-cleaned biological oxygen demand (BOD) bottle with 100 mL activated sludge from the SBR and 400 mL pre-aerated synthetic wastewater. The BOD bottle was airproofed using a rubber stopper with an oxygen-sensing probe. The DO concentration was recorded by the oxygen-sensing probe at intervals of 30 s. The mixed liquor in the BOD bottle was mixed with a magnetic stirrer. The MLSS in the BOD bottle was regarded as invariant during the whole experiment due to the shorter operational time. The SOUR was calculated from the DO–time curve based on the MLSS in the BOD bottle.The determination of SAOR, SNOR and SNRR was performed in a 500 mL Erlenmeyer flask with 100 mL activated sludge from the SBR and 350 mL synthetic wastewater. The activated sludge and synthetic mariculture wastewater in the Erlenmeyer flask was mixed with a magnetic stirrer. The nitrogen sources were added to respective samples of activated sludge and synthetic wastewater to determine SAOR, SNOR and SNRR. The nitrogen sources for the determination of SAOR, SNOR and SNRR were NH4Cl, NaNO2 and NaNO3, respectively, corresponding to the concentration of 120 mg L−1 NH4+-N, 147 mg L−1 NO2−-N and 303 mg L−1 NO3−-N. Air was introduced into the Erlenmeyer flask via an air diffuser in the determination of SAOR or SNOR, whereas nitrogen gas was used fill the Erlenmeyer flask for anaerobic conditions in the SNNR test. The MLSS in the Erlenmeyer flask was regarded as invariant during the determination of SAOR, SNOR and SNRR due to the shorter operational time. The SAOR, SNOR and SNRR were calculated by monitoring the decreased rate of NH4+-N, NO2−-N and NO3−-N versus time, respectively.
Microbial community analysis
Denaturing gradient gel electrophoresis (DGGE) was used to evaluate the microbial community of the activated sludge at different Cu(II) concentrations. DNA extraction, DGGE and sequencing were performed by following the methodology reported by Wang et al.31 The DNA was extracted from activated sludge using a PowerSoil@DNA Isolation Kit (Anbisheng Inc., China) according to the manufacturer's protocol.PCR amplifications were carried out in an iCycler Thermal Cycler PCR (Bio-Rad Co., Ltd., USA). The bacterial primer 8/20 F with a GC clamp (5′-CGCCCGCCGCGCGCGGCGGGCGGGGCGGGGGCACGGGGGGAGAGTTTGATCCTGGCTCAG-3′) and the universal primer 534 R (5′-ATT ACC GCG GCT GCT GG-3′), which targets the 16S rRNA V3 variable region, were used to amplify 16S rDNA.32 The 50 μL reaction mixture contained 2 μL extracted DNA template, 0.5 μL TaKaRa Taq™ DNA polymerase (5 U μL−1, TaKaRa Biotechnology Dalian Co. Ltd., China), 5 μL 10× PCR buffer (Mg2+ plus, TaKaRa Biotechnology Dalian Co. Ltd., China), 4 μL dNTP (each 2.5 mmol L−1), 1 μL of each primer (each 20 μmol L−1, TaKaRa Biotechnology Dalian Co. Ltd., China) and was adjusted to a final volume of 50 μL with sterile deionized water. The PCR amplification of 16S rDNA was performed according to the Touchdown PCR program: initial denaturation at 95 °C for 5 min, denaturation at 95 °C for 30 s, annealing at 56 °C for 30 s (the temperature was decreased by 0.5 °C every cycle until the touchdown temperature of 51 °C was reached), extension at 72 °C for 45 s, denaturation at 95 °C for 30 s after 10 cycles, annealing at 51 °C for 30 s, extension at 72 °C for 45 s, followed by a final extension at 72 °C for 10 min after 20 cycles and ending at 4 °C. The PCR products were stained with ethidium bromide (EB) and electrophoresed in a 1.2% (w/v) agarose gel at 120 V for 40 min. They were quantified by comparison with a standard marker (DL 2000, TaKaRa Biotechnology Dalian Co. Ltd., China).
DGGE was performed using a DCode™ Universal Mutation Detection System (Bio-Rad Co., Ltd., USA). PCR samples containing 40 μL PCR amplification products and 8 μL 6× loading buffer were loaded into each well of a 8% (w/v, g mL−1) polyacrylamide (37.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, acrylamide
1, acrylamide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bisacrylamide) gel in 1× TAE buffer (Tris–acetate–EDTA buffer) using a denaturing gradient ranging from 30 to 60% (30% denaturant contained 2.52 g urea, 2.4 mL deionized formamide, 4 mL 40% polyacrylamide and 0.4 mL 50 × TAE buffer, and was adjusted to a final volume of 20 mL with deionized water; the content of each component of the 60% denaturant was twice that of the 30%). Electrophoreses were performed at 65 °C and 150 V for 420 min. After electrophoresis, the gel was stained with 0.1% AgNO3 for 25 min and then rinsed with Milli-Q water. The gels were visualized under UV light with the Gel Doc XR System (Bio-Rad Co., Ltd., USA).
bisacrylamide) gel in 1× TAE buffer (Tris–acetate–EDTA buffer) using a denaturing gradient ranging from 30 to 60% (30% denaturant contained 2.52 g urea, 2.4 mL deionized formamide, 4 mL 40% polyacrylamide and 0.4 mL 50 × TAE buffer, and was adjusted to a final volume of 20 mL with deionized water; the content of each component of the 60% denaturant was twice that of the 30%). Electrophoreses were performed at 65 °C and 150 V for 420 min. After electrophoresis, the gel was stained with 0.1% AgNO3 for 25 min and then rinsed with Milli-Q water. The gels were visualized under UV light with the Gel Doc XR System (Bio-Rad Co., Ltd., USA).
The DGGE profiles were analyzed using the Quantity One software (version 4.6.2, Bio-Rad Co., Ltd., USA). Dendrograms relating band pattern similarities were automatically calculated using the unweighted pair-group method with arithmetic average (UPGMA) clustering algorithm, which was included in the Quantity One software.
The DGGE bands were excised from the gel, re-amplified and electrophoresed again in DGGE gel to confirm the mobility of the bands. The new PCR products were cloned. The cloned products were sequenced by Shanghai Jinsirui Biological Science and Technology Co., Ltd. Bacteria serial numbers were obtained from the ribosomal database project (http://rdp.cme.msu.edu/). The sequences were compared using the BLAST search option in the NCBI nucleotide sequence database (http://www.ncbi.nlm.nih.gov/).
Results and discussion
Effects of OTC on COD and nitrogen removal of the anoxic–aerobic SBR
Fig. 1 shows the variations in the COD, NH4+-N, NO2-N and NO3−-N concentrations in the influent and effluent at different OTC concentrations. The average COD and NH4+-N removal efficiencies under steady states slowly decreased from 98.1% and 91.5% to 95.3% and 90.9% with the increase in OTC concentration from 0 to 2 mg L−1, respectively. However, the average COD removal efficiencies under steady states drastically decreased from 95.3% to 56.5% with the increase in OTC concentration from 2 to 12 mg L−1, suggesting that high OTC concentration could obviously inhibit the microbial activities of activated sludge in the anoxic–aerobic SBR. As shown in Fig. 1b, the NH4+-N concentration in the effluent increased dramatically with the increase in OTC concentration from 2 to 12 mg L−1, indicating that ammonia oxidizing bacteria are sensitive to OTC. This result is consistent with those from the SAOR test. Katipoglu-Yazan et al.33 also reported the similar result that the nitrification process could be inhibited at high OTC concentration. On the contrary, Campos et al.34 found that nitrification could remain uninhibited with the increase in OTC concentration from 0 to 100 mg L−1. As shown in Fig. 1c and d, no apparent NO3−-N accumulation was found during the whole operational period, whereas NO2−-N accumulation occurred at 12 mg L−1 OTC, which could be explained by the fact that high OTC concentration may inhibit the oxidization of NO2−-N into NO3−-N in the nitrification process or the reduction of NO2−-N into N2 in the denitrification process.Effects of OTC on SOUR, SAOR, SNOR and SNNR of activated sludge
To investigate the effects of OTC on the microbial activities of activated sludge in the anoxic–aerobic SBR, the SOUR, SAOR, SNOR and SNNR of the activated sludge under steady states were analyzed at different OTC concentrations in the influent. As shown in Fig. 2a, the SOUR of the activated sludge gradually decreased from 32.5 to 9.3 mg O2 g−1 MLSS h−1 with the increase in OTC concentration from 0 to 12 mg L−1. Compared to OTC-free addition of influent, the SOUR of the activated sludge decreased by 71.1% at 12 mg L−1 OTC, suggesting that the addition of OTC in the influent could obviously affect the respiration and catabolic activity of heterotrophic bacteria. Kolar et al.35 reported that OTC showed obvious inhibition of respiration of activated sludge. As shown in Fig. 2b, the SAOR and SNOR gradually decreased from 2.5 and 3.08 mg N g−1 MLSS h−1 to 0.3 and 0.9 mg N g−1 MLSS h−1 with the increase in OTC concentration from 0 to 12 mg L−1, respectively. The SAOR and SNOR declined by 88% and 71% at 12 mg L−1 OTC compared to 0 mg L−1 OTC, respectively, indicating that the SAOR decreased at a faster rate than the SNOR with the increase in OTC concentration. The present results showed that OTC exerted more inhibitory effects on ammonia oxidation than nitrite oxidation in the anoxic–aerobic SBR treating mariculture wastewater. Halling-Sørensen et al.36 reported that OTC could inhibit the nitrification rate of activated sludge, whereas they did not compare the extent of the effect of OTC on SAOR and SNOR. The SNNR of activated sludge gradually decreased from 27.6 to 13.0 mg N g−1 MLSS h−1 with the increase in OTC concentration from 0 to 12 mg L−1 (Fig. 2c). The SNNR values were always higher than the sum of the SAOR and SNOR at different OTC concentrations, which could explain the phenomena of no obvious accumulation of NO3−-N and NO2−-N in the present study. | ||
| Fig. 2 Effect of OTC on microbial activity of activated sludge: (a) SOUR; (b) SAOR and SNOR; and (c) SNRR. | ||
Effects of OTC on PS and PN of LB-EPS and TB-EPS from activated sludge
EPS are mainly composed of polysaccharides (PS), protein (PN), and small amounts of nucleic acids and lipids, which can affect floc structure, surface charge, flocculation, settleability and adhesion ability of activated sludge in biological wastewater treatment systems. In order to evaluate the effect of OTC on the EPS, the variation of PN and PS contents in the LB-EPS and TB-EPS were analyzed at different OTC concentrations (Fig. 3). The contents of LB-EPS and TB-EPS increased with increasing OTC concentration in the influent due to the toxicity response of the microorganisms to OTC. Due to the increase in LB-EPS and TB-EPS contents, the SVI under steady states increased from 55 to 118 mL g−1 with the increase in OTC concentration from 0 to 12 mg L−1. Huang et al.37 reported the similar result that the EPS content of activated sludge from a SBR increased with the increase in tetracycline (TC) concentration from 0 to 100 μg L−1. As shown in Fig. 3a, the PN and PS contents in the LB-EPS increased from 6.2 and 5.5 mg g−1 MLVSS to 29.8 and 19.6 mg g−1 MLVSS with the increase in OTC concentration from 0 to 12 mg L−1, respectively. Similarly, the PN and PS contents in the TB-EPS increased from 8.4 and 5.2 mg g−1 MLVSS to 34.3 and 17.4 mg g−1 MLVSS with the increase of OTC concentration from 0 to 12 mg L−1, respectively. The PN contents in LB-EPS and TB-EPS were always higher that the PS contents at different OTC concentrations. In addition, the PN/PS ratios of both the LB-EPS and TB-EPS showed no obvious variation at 0–8 mg L−1, whereas they showed a slight increase at 12 mg L−1 (Fig. 3c). The PN/PS ratio in the TB-EPS was always higher than that in the LB-EPS at different OTC concentrations, which could be explained by the fact that the PN in TB-EPS was more sensitive to the increase in OTC concentration than that in LB-EPS. Shi et al.38 also reported that the PN content of EPS in granular sludge was greater than the PS content under tetracycline (TC) stress.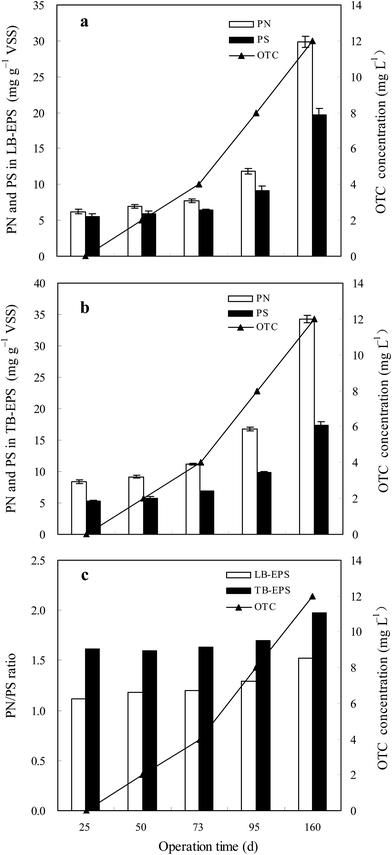 | ||
| Fig. 3 Effect of OTC on PN and PS in TB-EPS and LB-EPS from sludge: (a) PN and PS in LB-EPS; (b) PN and PS in TB-EPS; and (c) PN/PS ratio. | ||
Effects of OTC on 3D-EEM fluorescence spectra of LB-EPS and TB-EPS
The 3D-EEM fluorescence spectra of LB-EPS and TB-EPS were investigated at 0, 2, 8 and 12 mg L−1 OTC. Each 3D-EEM fluorescence spectrum provides special information about the chemical compositions of LB-EPS and TB-EPS. As shown in Fig. 4, Peaks A and B in both the LB-EPS and TB-EPS were located at the excitation/emission wavelengths (Ex/Em) of 280/330–340 nm and 220–230/330–340 nm, respectively. The two peaks were separately assigned to tryptophan protein-like substances (Peak A) and aromatic protein-like substances (Peak B).39,40 Peaks C and D in the LB-EPS and TB-EPS were found at (Ex/Em) of 270/430–440 nm and 350–360/450 nm, respectively. Peak C was attributed to fulvic acid-like substances, and Peak D was related to humic acid-like substances.39 Both Peaks A and B always existed in the LB-EPS and TB-EPS at different OTC concentrations. Peak C in the LB-EPS appeared at 2, 8 and 12 mg L−1 OTC, and Peak D in the LB-EPS was found at 8 and 12 mg L−1 OTC. Peaks C and D in the TB-EPS appeared at 8 mg L−1 OTC, whereas Peak D in the TB-EPS disappeared at 12 mg L−1 OTC. The appearance or disappearance of different fluorescence peaks showed the variation in the chemical composition in the LB-EPS and TB-EPS with increasing OTC concentration in the influent.Some fluorescence peaks shifted toward either longer (red shift) or shorter (blue shift) wavelengths with the addition of OTC in the influent, depending on the emission and/or excitation scale. The location and fluorescence intensity of the 3D-EEM fluorescence spectra at different OTC concentrations are summarized in Table 1. Compared to 0 mg L−1 OTC, the location of Peak A in the LB-EPS was blue-shifted by 10 nm along the Em axis at 8 and 12 mg L−1 OTC. However, the location of Peak B in the LB-EPS showed no shift with the increase in OTC concentration from 0 to 12 mg L−1. The location of Peak B in the LB-EPS was blue-shifted by 0/10, 10/10 and 10/0 nm along the Ex/Em axis at 2, 8 and 12 mg L−1 OTC, respectively. Compared to 8 mg L−1 OTC, the location of Peak C in the TB-EPS was blue-shifted by 10 nm along the Em axis at 12 mg L−1 OTC, and the location of Peak D in both the LB-EPS and TB-EPS was blue-shifted by 10 nm along the Em axis at 12 mg L−1 OTC. The blue shifts in the LB-EPS and TB-EPS were reported to be associated with a decomposition of condensed aromatic moieties and the break-up of the large molecules into fragments.41 The red shift of the fluorescence peak in the LB-EPS and TB-EPS could be attributed to the presence of carbonyl-containing substituents, hydroxyl, alkoxyl and amino groups, and carboxyl constituents.39 In addition, the variations in the fluorescence peak intensity could indicate the variations in the chemical compositions of LB-EPS and TB-EPS with increasing OTC concentration.
| Samples | OTC (mg L−1) | Peak A | Peak B | Peak C | Peak D | ||||
|---|---|---|---|---|---|---|---|---|---|
| Ex/Em | Intensity | Ex/Em | Intensity | Ex/Em | Intensity | Ex/Em | Intensity | ||
| LB-EPS | 0 | 280/340 | 225 | 230/340 | 225.4 | — | — | — | — |
| 2 | 280/340 | 121.9 | 230/330 | 150.7 | 270/440 | 46.1 | — | — | |
| 8 | 280/330 | 171.8 | 220/330 | 178.1 | 270/440 | 45.78 | 360/450 | 21.56 | |
| 12 | 280/330 | 230.8 | 220/340 | 184.6 | 270/440 | 62.04 | 350/450 | 28.86 | |
| TB-EPS | 0 | 280/340 | 281.8 | 220/340 | 297.8 | — | — | — | — |
| 2 | 280/340 | 52.76 | 220/340 | 68.08 | — | — | — | — | |
| 8 | 280/340 | 206.3 | 230/340 | 134.6 | 270/440 | 83.65 | 360/450 | 38.09 | |
| 12 | 280/340 | 176.8 | 220/340 | 354.9 | 270/430 | 27.25 | 350/450 | 7.316 | |
Effects of OTC on FTIR spectra of LB-EPS and TB-EPS
FTIR spectra were analyzed in the region of 4000–400 cm−1 to identify the main functional groups in the LB-EPS and TB-EPS at different OTC concentrations. As shown in Fig. 5, the broad absorption peaks between 3417 and 3450 cm−1 in both the LB-EPS and TB-EPS were indicative of the existence of the O–H groups of glucose and the N–H groups of proteins,42 and their relative intensities decreased with the increase in OTC concentration from 0 to 12 mg L−1. The absorption peaks around 1640 cm−1 in both the LB-EPS and TB-EPS were correlated with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of the β-sheets in secondary protein structures.43 The intensities of the absorption peaks around 1640 nm also decreased with the increase in OTC from 0 to 12 mg L−1. The relative intensities and location of the absorption bands near 1400 cm−1 in both the LB-EPS and TB-EPS exhibited obvious variations with the increase in OTC from 0 to 12 mg L−1, and are associated with the C–O and COO− stretching of carboxylic groups in proteins.44,45 The absorption peaks near 1100 cm−1 in both the LB-EPS and TB-EPS were related to the O–C–O stretching vibrations in polysaccharides,46 and their relative intensity increased with the increase in OTC from 0 to 12 mg L−1. The absorption peaks between 597 and 617 cm−1 indicated that unsaturated bonds existed in both the LB-EPS and TB-EPS. The variations in peak intensity and location in the FTIR spectra showed that the addition of OTC in the influent had some obvious effects on the functional groups of PN and PS in the LB-EPS and TB-EPS.
O stretching vibration of the β-sheets in secondary protein structures.43 The intensities of the absorption peaks around 1640 nm also decreased with the increase in OTC from 0 to 12 mg L−1. The relative intensities and location of the absorption bands near 1400 cm−1 in both the LB-EPS and TB-EPS exhibited obvious variations with the increase in OTC from 0 to 12 mg L−1, and are associated with the C–O and COO− stretching of carboxylic groups in proteins.44,45 The absorption peaks near 1100 cm−1 in both the LB-EPS and TB-EPS were related to the O–C–O stretching vibrations in polysaccharides,46 and their relative intensity increased with the increase in OTC from 0 to 12 mg L−1. The absorption peaks between 597 and 617 cm−1 indicated that unsaturated bonds existed in both the LB-EPS and TB-EPS. The variations in peak intensity and location in the FTIR spectra showed that the addition of OTC in the influent had some obvious effects on the functional groups of PN and PS in the LB-EPS and TB-EPS.
 | ||
| Fig. 5 FTIR spectra of LB-EPS and TB-EPS at different OTC concentrations: (a) LB-EPS and (b) TB-EPS. | ||
XPS analysis of LB-EPS and TB-EPS at different OTC concentrations
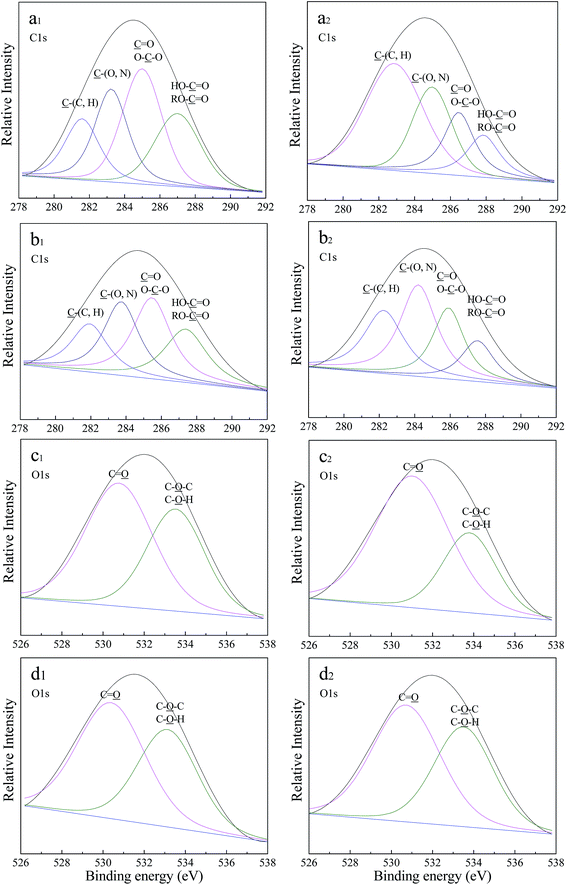
Table 2 summarizes the elemental compositions of LB-EPS and TB-EPS at 0 and 12 mg L−1 OTC through XPS analysis, resulting from integrating the C1s, O1s and N1s. The variation in binding energy was mainly displayed in the O1s and N1s with the addition of OTC in the influent. Nitrogen appeared at the binding energies of 399.4 and 399.6 eV for LB-EPS and TB-EPS at 0 mg L−1 OTC, respectively, which were related to the amine or amide groups of proteins.47 The results were in agreement with the FTIR spectra (adsorption bands at 1635–1645 cm−1). Compared to 0 mg L−1 OTC in the influent, the binding energy of N1s for LB-EPS at 12 mg L−1 OTC indicated a shift of 1.2 eV towards the higher energy region, and the binding energy of N1s for TB-EPS shifted 0.4 eV towards the lower energy region. The binding energy of O1s for TB-EPS at 12 mg L−1 was 0.4 eV higher than that of O1s at 0 mg L−1, whereas the binding energy of O1s showed no obvious variation.| Element | LB-EPS | TB-EPS | Assignments | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Peaka (eV) | Atomia (%) | Peakb (eV) | Atomib (%) | Peaka (eV) | Atomia (%) | Peakb (eV) | Atomib (%) | ||
| a The XPS data was obtained at OTC concentration of 0 mg L−1.b The XPS data was obtained at OTC concentration of 12 mg L−1. | |||||||||
| Total C | 284.6 | 56.9 | 284.6 | 63.6 | 284.6 | 65.0 | 284.6 | 66.5 | |
| C1s | 281.6 | 17.4 | 282.9 | 44.8 | 281.9 | 23.3 | 282.3 | 26.6 | C–(C, H) |
| C1s | 283.2 | 25.4 | 285.0 | 24.6 | 283.7 | 31.6 | 284.2 | 35.6 | C–(O, N) |
| C1s | 285.0 | 33.2 | 286.5 | 17.8 | 285.5 | 26.8 | 285.9 | 23.6 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O,O–C–O O,O–C–O |
| C1s | 287.0 | 24.0 | 287.9 | 12.8 | 287.4 | 18.3 | 287.6 | 14.2 | HO–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, RO–C O, RO–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
| Total N | 399.4 | 9.9 | 400.6 | 3.0 | 399.6 | 3.2 | 399.2 | 2.9 | |
| Total O | 532.0 | 33.2 | 532.0 | 33.4 | 531.6 | 31.8 | 532.0 | 30.6 | |
| O1s | 530.8 | 41.5 | 531.0 | 69.5 | 530.4 | 57.4 | 530.7 | 57.4 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
| O1s | 533.5 | 58.5 | 533.8 | 30.5 | 533.1 | 42.6 | 533.5 | 42.6 | C–O–C,C–O–H |
The high-resolution spectra of the C1s and O1s regions are shown in Fig. 6, and the assignment and quantification of these XPS peaks are listed in Table 2. The carbon peak (C1s) in both LB-EPS and TB-EPS at 0 mg L−1 OTC can be decomposed into four peaks as follows: (1) the peaks at 281.6 and 281.9 eV are related to C–(C, H) from the side chains of lipids or amino groups; (2) the peaks at 283.2 and 283.7 eV are assigned to C–(O, N) from amine, alcohol or amide groups of proteins; (3) the peaks at 285.0 and 285.5 eV are attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O or O–C–O in amide, carbonyl, carboxylate, ester, acetal or hemiacetal groups; and (4) the peaks at 287.0 and 287.4 eV are ascribed to HO–C
O or O–C–O in amide, carbonyl, carboxylate, ester, acetal or hemiacetal groups; and (4) the peaks at 287.0 and 287.4 eV are ascribed to HO–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O or RO–C
O or RO–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O from ester or carboxyl groups. The high-resolution spectra of C1s in both LB-EPS and TB-EPS at 12 mg L−1 were similar to those at 0 mg L−1. Some obvious variations in the relative intensities of the C1s peak in LB-EPS and TB-EPS were found in Fig. 6, and the binding energy of each peak increased with the addition of OTC. The peaks for C
O from ester or carboxyl groups. The high-resolution spectra of C1s in both LB-EPS and TB-EPS at 12 mg L−1 were similar to those at 0 mg L−1. Some obvious variations in the relative intensities of the C1s peak in LB-EPS and TB-EPS were found in Fig. 6, and the binding energy of each peak increased with the addition of OTC. The peaks for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (or O–C–O) in both LB-EPS and TB-EPS were the largest peaks at 0 mg L−1 OTC. However, the largest peaks in LB-EPS and TB-EPS at 12 mg L−1 OTC were related to C–(C, H) and C–(O, N), respectively. In addition, the O1s peaks in both LB-EPS and TB-EPS at 0 mg L−1 OTC were decomposed to two peaks as follows: (1) the peaks at 530.8 and 530.4 eV are related to C
O (or O–C–O) in both LB-EPS and TB-EPS were the largest peaks at 0 mg L−1 OTC. However, the largest peaks in LB-EPS and TB-EPS at 12 mg L−1 OTC were related to C–(C, H) and C–(O, N), respectively. In addition, the O1s peaks in both LB-EPS and TB-EPS at 0 mg L−1 OTC were decomposed to two peaks as follows: (1) the peaks at 530.8 and 530.4 eV are related to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O from carbonyl, carboxylate, amide or ester groups; and (2) the peaks at 533.5 and 533.1 eV were assigned to C–O–C or C–O–H from alcohol, hemiacetal or acetal groups, which indicates the presence of polysaccharides. Similarly, the addition of OTC led to variation in the relative intensity and binding energy of each O1s peak. Compared to 0 mg L−1 OTC, the percentages of each O1s in the total O1s increased or decreased in LB-EPS at 12 mg L−1 OTC, whereas those in TB-EPS showed no variation at 12 mg L−1 OTC. It could be concluded that the response mechanism to OTC toxicity for LB-EPS was different to that of TB-EPS. Xu et al.48 reported that the interaction of biodegradation, adsorption and hydrophobicity was the major mechanism for the interaction of EPS and antibiotics. The XPS results illustrated that amino, carboxyl and hydroxyl groups in the EPS might be involved in interaction between the EPS and OTC. Some studies reported that the hydroxyl group was an active group for antibiotic activity, and it could affect the biotoxicity of antibiotics and the inhibitory effect on microorganisms.49,50
O from carbonyl, carboxylate, amide or ester groups; and (2) the peaks at 533.5 and 533.1 eV were assigned to C–O–C or C–O–H from alcohol, hemiacetal or acetal groups, which indicates the presence of polysaccharides. Similarly, the addition of OTC led to variation in the relative intensity and binding energy of each O1s peak. Compared to 0 mg L−1 OTC, the percentages of each O1s in the total O1s increased or decreased in LB-EPS at 12 mg L−1 OTC, whereas those in TB-EPS showed no variation at 12 mg L−1 OTC. It could be concluded that the response mechanism to OTC toxicity for LB-EPS was different to that of TB-EPS. Xu et al.48 reported that the interaction of biodegradation, adsorption and hydrophobicity was the major mechanism for the interaction of EPS and antibiotics. The XPS results illustrated that amino, carboxyl and hydroxyl groups in the EPS might be involved in interaction between the EPS and OTC. Some studies reported that the hydroxyl group was an active group for antibiotic activity, and it could affect the biotoxicity of antibiotics and the inhibitory effect on microorganisms.49,50
Effects of OTC on microbial community of SBR
The microbial communities of activated sludge in the anoxic–aerobic SBR were investigated at different OTC concentrations in the influent. As shown in Fig. 5a, the DGGE profile and band intensity displayed some obvious variation with the increase in OTC concentration from 0 to 12 mg L−1. The bands (e.g. bands 2–5) in the DGGE profile were always predominant at different OTC concentrations. The intensities of some bands (e.g. bands 6, 15 and 29) increased with increasing OTC concentration, whereas other bands (e.g. bands 14, 25 and 30) gradually weakened or disappeared at relatively high OTC concentration. The similarities in the microbial communities at different OTC concentrations were analyzed by using UPGMA clustering analysis. As shown in Fig. 7b, the sludge sample at 0 mg L−1 OTC was assigned to the first group and the sludge samples at 2–12 mg L−1 OTC were classified as the second group. The similarity between the first group and the second group was 0.80, which suggested that the addition of OTC in the influent could affect the microbial community of activated sludge in the anoxic–aerobic SBR. The second group was divided into different subgroups, and the similarity between the two neighboring subgroups showed some variations due to the increase in OTC concentration from 2 to 12 mg L−1. | ||
| Fig. 7 DGGE analysis of microbial communities of activated sludge in the anoxic–aerobic SBR at different OTC concentrations: (a) DGGE profile and (b) cluster analysis based on UPGMA analysis. | ||
To further analyze the variation in the microbial community with increasing OTC concentration, thirty-three discernable bands were excised from the DGGE gel and then sequenced. The closest relative strains available in the GenBank were obtained by using BLAST program (Table 3). Some bacteria were always the predominant species in the activated sludge at different OTC concentrations, such as Photobacterium sp. 384 (band 2), Photobacterium halotolerans (band 3), uncultured Anaerolineaceae bacterium (band 4) and Maricaulis maris (band 5), suggesting these bacteria could tolerate the toxicity of 12 mg L−1 OTC in the influent. Rivas et al.51 reported that Photobacterium halotolerans had the ability to tolerate high salt concentrations and reduce nitrate to nitrite under anaerobic conditions. Some bacteria, e.g. Hyphomonas jannaschiana (band 6), uncultured bacterium (band 15), Denitromonas aromaticus (band 26) and Denitromonas sp. D1-68 (band 29), gradually became the dominant species with the increase in OTC concentration from 0 to 12 mg L−1. Weiner et al.52 reported that Hyphomonas jannaschiana could degrade aromatic compounds, which might be related to the degradation of OTC in the present study. Some researchers reported that Denitromonas aromaticus and Denitromonas sp. D1-68 could reduce nitrate to nitrogen gas in the denitrification process.53,54 Denitratisoma oestradiolicum (band 14) and Phyllobacterium myrsinacearum (25) gradually disappeared with increasing OTC concentration, indicating that they could not tolerate the toxicity of the high OTC concentrations. Fahrbach et al.55 reported that Denitratisoma oestradiolicum could use nitrate as an electron acceptor under anaerobic conditions. Micropruina glycogenica (band 10) can reduce nitrate to nitrite under anaerobic conditions and utilize glucose as carbon resource.56
| Bands | Closest related sequences | Accession number | Similarity (%) | Class containing related sequences |
|---|---|---|---|---|
| 1 | Azoarcus tolulyticus | AF123075 | 92 | β-Proteobacteria |
| 2 | Photobacterium sp. 384 | JQ012981 | 96 | γ-Proteobacteria |
| 3 | Photobacterium halotolerans (T) | AY551089 | 98 | γ-Proteobacteria |
| 4 | Uncultured Anaerolineaceae bacterium | JN038290 | 98 | Anaerolineae |
| 5 | Maricaulis maris | AJ227803 | 95 | α-Proteobacteria |
| 6 | Hyphomonas jannaschiana | KF863146 | 98 | δ-Proteobacteria |
| 7 | Marinicella sp. U1369-101122-SW153 | JQ082140 | 97 | γ-Proteobacteria |
| 8 | Marinicella litoralis (T) | AB500095 | 95 | γ-Proteobacteria |
| 9 | Delftia acidovorans | AB074256 | 100 | β-Proteobacteria |
| 10 | Micropruina glycogenica (T) | AB012607 | 98 | Actinobacteria |
| 11 | Sedimenticola selenatireducens | NR041877 | 98 | γ-Proteobacteria |
| 12 | Marinicella litoralis | HQ908724 | 94 | γ-Proteobacteria |
| 13 | Clostridium sp. CM 19 | FJ155851 | 98 | Clostridia |
| 14 | Denitratisoma oestradiolicum | KF810118 | 99 | β-Proteobacteria |
| 15 | Uncultured bacterium | HQ506904 | 99 | – |
| 16 | Donghicola xiamenensis (T) | DQ120728 | 96 | α-Proteobacteria |
| 17 | Paracoccus denitrificans | KF740559 | 98 | α-Proteobacteria |
| 18 | Roseovarius halotolerans (T) | EU431217 | 100 | α-Proteobacteria |
| 19 | Mesorhizobium loti | X67230 | 98 | α-Proteobacteria |
| 20 | Thermobrachium celere (T) | X99238 | 99 | Clostridia |
| 21 | Lutimaribacter litoralis | AB627076 | 99 | γ-Proteobacteria |
| 22 | Photobacterium ganghwense (T) | AY960847 | 96 | γ-Proteobacteria |
| 23 | Denitromonas indolicum | AY972852 | 98 | β-Proteobacteria |
| 24 | Halomonas sp. PS21(2010) | GU930762 | 99 | γ-Proteobacteria |
| 25 | Phyllobacterium myrsinacearum | D12789 | 98 | γ-Proteobacteria |
| 26 | Denitromonas aromaticus | AB049763 | 98 | β-Proteobacteria |
| 27 | Paracoccus sp. DEH99 | FJ713782 | 100 | α-Proteobacteria |
| 28 | Paracoccus homiensis (T) | DQ342239 | 99 | α-Proteobacteria |
| 29 | Denitromonas sp. D1-68 | AM403170 | 98 | β-Proteobacteria |
| 30 | Hyphomicrobium nitrativorans | JX131369 | 98 | α-Proteobacteria |
| 31 | Brenneria rubrifaciens | EU490604 | 99 | γ-Proteobacteria |
| 32 | Brevundimonas sp. p22P | EF486314 | 100 | α-Proteobacteria |
| 33 | Methylibium sp. UKPF16 | AB769223 | 99 | β-Proteobacteria |
Conclusions
The performance and microbial community of activated sludge at different OTC concentrations were investigated in an anoxic–aerobic SBR treating mariculture wastewater. The COD and nitrogen removal, SOUR, SAOR, SNOR and SNRR decreased with increasing OTC concentration. No apparent NO3−-N accumulation was found during the whole operational period, whereas NO2−-N accumulation occurred at high OTC concentration. The PN and PS contents in the LB-EPS and TB-EPS increased with increasing OTC concentration, and the functional groups of PN and PS could be affected by the appearance of OTC in the influent. Some amino, carboxyl and hydroxyl groups in the LB-EPS and TB-EPS might be involved in the interaction between EPS and OTC. The microbial communities in the SBR showed obvious variation with increasing OTC concentration. Some microorganisms were depleted or weakened under OTC stress, whereas others gradually became the predominant microorganisms due to their ability to adapt to the OTC toxicity.Acknowledgements
The work was funded by the National Natural Science Foundation of China (no. 51178437).References
- V. Jegatheesan, C. Zeng, L. Shu, C. Manicom and C. Steicke, J. Cleaner Prod., 2007, 15, 1535 CrossRef PubMed.
- T. Pulefou, V. Jegatheesan, C. Steicke and S. H. Kim, Desalination, 2008, 221, 534 CrossRef CAS PubMed.
- M. T. Gutierrez-Wing and R. F. Malone, Aquacult. Eng., 2006, 34, 163 CrossRef PubMed.
- M. Shpigel, D. Ben-Ezra, L. Shauli, M. Sagi, Y. Ventura, T. Samocha and J. J. Lee, Aquaculture, 2013, 412–413, 52 CrossRef PubMed.
- D. Roy, K. Hassan and R. Boopathy, J. Ind. Microbiol. Biotechnol., 2010, 37, 1105 CrossRef CAS PubMed.
- S. P. Gregory, R. J. Shields, D. J. Fletcher, P. Gatland and P. J. Dyson, Ecol. Eng., 2010, 36, 1485 CrossRef PubMed.
- B. L. Brazil, Aquacult. Eng., 2006, 34, 261 CrossRef PubMed.
- M. Seoane, C. Rioboo, C. Herrero and Á. Cid, Mar. Environ. Res., 2014, 101, 1 CrossRef CAS PubMed.
- K. Grigorakis and G. Rigos, Chemosphere, 2011, 855, 899 CrossRef PubMed.
- F. C. Cabello, Environ. Microbiol., 2006, 8, 1137 CrossRef CAS PubMed.
- J. Näslund, J. E. Hedman and C. Agestrand, Aquat. Toxicol., 2008, 90, 223 CrossRef PubMed.
- L. A. Cardoza, C. W. Knapp, C. K. Larive, J. B. Belden, M. Lydy and D. W. Graham, Water, Air, Soil Pollut., 2005, 161, 383 CrossRef CAS.
- I. M. Hagenbuch and J. L. Pinckney, Water Res., 2012, 46, 5028 CrossRef CAS PubMed.
- Q. Zheng, R. J. Zhang, Y. H. Wang, X. H. Pan, J. H. Tang and G. Zhang, Mar. Environ. Res., 2012, 78, 26 CrossRef CAS PubMed.
- A. Gonzalez-Martinez, A. Rodriguez-Sanchez, M. V. Martinez-Toledo, M. J. Garcia-Ruiz, E. Hontoria, F. Osorio-Robles and J. Gonzalez-Lopez, Sci. Total Environ., 2014, 476–477, 276 CrossRef CAS PubMed.
- S. Aydin, Z. Cetecioglu, O. Arikan, B. Ince, E. G. Ozbayram and O. Ince, Chemosphere, 2015, 120, 515 CrossRef CAS PubMed.
- J. Y. Ji, Y. J. Xing, Z. T. Ma, M. Zhang and P. Zheng, Chemosphere, 2013, 246, 319 Search PubMed.
- T. Shimada, J. L. Zilles, E. Morgenroth and L. Raskin, Biotechnol. Bioeng., 2008, 101, 73 CrossRef CAS PubMed.
- J. Y. Ji, Y. J. Xing, Z. T. Ma, J. Cai, P. Zheng and H. F. Lu, J. Hazard. Mater., 2013, 246–247, 319 CrossRef CAS PubMed.
- H. Chen, S. Liu, X. R. Xu, S. S. Liu, G. J. Zhou, K. F. Sun, J. L Zhao and G. G. Ying, Mar. Pollut. Bull., 2015, 90, 181 CrossRef CAS PubMed.
- G. M. Lalumera, D. Calamari, P. Galli, S. Castiglioni, G. Crosa and R. Fanelli, Chemosphere, 2004, 54, 661 CrossRef CAS PubMed.
- D. Schnappinger and W. Hillen, Arch. Microbiol., 1996, 165, 359 CrossRef CAS.
- Y. L. Liu and X. L. Pan, Huanjing Kexue Yu Jishu, 2012, 6, 51 Search PubMed.
- G. P. Sheng, H. Q. Yu and X. Y. Li, Biotechnol. Bioeng., 2006, 20, 1095 CrossRef PubMed.
- F. S. Wei, W. Q. Qi, T. Bi, Z. G. Sun, Y. R. Huang and Y. W. Shen, Water and Wastewater Monitoring Methods, Chinese Environmental Science Publishing Press, Beijing, China, 2002 Search PubMed.
- Z. C. Wang, M. C. Gao, Z. Wang, Z. L. She, Q. B. Chang, C. Q. Sun, J. Zhang, Y. Ren and N. Yang, Chemosphere, 2013, 93, 2789 CrossRef CAS PubMed.
- O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall, J. Biol. Chem., 1951, 193, 265 CAS.
- M. Dubois, K. A. Gilles, J. K. Hamilton, P. A. Rebers and F. Smith, Anal. Chem., 1956, 28, 350 CrossRef CAS.
- Z. C. Wang, M. C. Gao, S. Wang, Y. J. Xin, D. Ma, Z. L. She, J. Zhang, Z. Wang, Q. B. Chang and Y. Ren, Chem. Eng. J., 2014, 251, 165 CrossRef CAS PubMed.
- S. Wang, M. C. Gao, Z. C. Wang, Z. L. She, C. J. Jin, Y. G. Zhao and Z. W. Li, RSC Adv., 2015, 5, 30737 RSC.
- Z. C. Wang, M. C. Gao, Y. Zhang, Z. L. She, Y. Ren, Z. Wang and C. C. Zhao, J. Environ. Manage., 2014, 142, 10 CrossRef CAS PubMed.
- G. Muyzer, E. C. de Waal and A. G. Uitterlinden, Appl. Environ. Microbiol., 1993, 59, 695 CAS.
- T. Katipoglu-Yazan, I. Pala-Ozkok, E. Ubay-Cokgor and D. Orhon, Bioresour. Technol., 2013, 144, 410–419 CrossRef CAS PubMed.
- J. L. Campos, J. M. Garrido, R. Méndez and J. M. Lema, Appl. Biochem. Biotechnol., 2001, 95, 1 CrossRef CAS.
- B. Kolar, L. Arnuš, B. Jeretin, A. Gutmaher, D. Drobne and M. K. Durjava, Chemosphere, 2014, 115, 75 CrossRef CAS PubMed.
- B. Halling-Sørensen, Arch. Environ. Contam. Toxicol., 2001, 40, 451 CrossRef.
- M. H. Huang, W. Zhang, Y. Zheng and W. Zhang, Chemosphere, 2014, 117, 658 CrossRef CAS PubMed.
- Y. Shi, S. Xing, X. Wang and S. Wang, Bioresour. Technol., 2013, 139, 170 CrossRef CAS PubMed.
- W. Chen, P. Westerhoff, J. Leenheer and K. Booksh, Environ. Sci. Technol., 2003, 37, 5701 CrossRef CAS.
- Y. Yamashita and E. Tanoue, Mar. Chem., 2003, 82, 255 CrossRef CAS.
- J. Świetlik, A. Dabrowska, U. Raczyk-Stanislawiak and J. Nawrocki, Water Res., 2004, 38, 547 CrossRef PubMed.
- Z. W. Liang, W. H. Li, S. Y. Yang and P. Du, Chemosphere, 2010, 81, 626 CrossRef CAS PubMed.
- Z. Wang, Z. Wu, X. Yin and L. Tian, J. Membr. Sci., 2008, 325, 238 CrossRef CAS PubMed.
- K. E. Eboigbodin and C. A. Biggs, Biomacromolecules, 2008, 9, 686 CrossRef CAS PubMed.
- B. Tesson, C. Gaillard and V. Martin-Jézéquel, Mar. Chem., 2008, 109, 60 CrossRef CAS PubMed.
- X. F. Sun, S. G. Wang, X. M. Zhang, J. Chen, X. M. Li, B. Y. Gao and Y. Ma, J. Colloid Interface Sci., 2009, 335, 11 CrossRef CAS PubMed.
- X. F. Sun, C. Liu, Y. Ma, S. G. Wang, B. Y. Gao and X. M. Li, Colloids Surf., B, 2011, 82, 456 CrossRef CAS PubMed.
- J. Xu, G. P. Sheng, Y. Ma, L. F. Wang and H. Q. Yu, Water Res., 2013, 47, 5298 CrossRef CAS PubMed.
- F. Schlünzen, R. Zarivach, J. Harms, A. Bashan, A. Tocilj, R. Albrecht, A. Yonath and F. Franceschi, Nature, 2001, 413, 814 CrossRef PubMed.
- C. Song, X. F. Sun, S. F. Xing, P. F. Xia, Y. J. Shi and S. G. Wang, Environ. Sci. Pollut. Res., 2014, 21, 1786 CrossRef CAS PubMed.
- R. Rivas, P. García-Fraila, P. F. Mateos, E. Martínez-Molina and E. Velázquez, Int. J. Syst. Evol. Microbiol., 2006, 56, 1067 CrossRef CAS PubMed.
- R. M. Weiner, R. A. Devine, D. M. Powell, L. Dagasan and R. L. Moore, Int. J. Syst. Evol. Microbiol., 1985, 35, 237 Search PubMed.
- J. P. Bassin, M. Pronk, G. Muyzer, R. Kleerebezem, M. Dezotti and M. C. M. van Loosdrecht, Appl. Environ. Microbiol., 2011, 77, 7942 CrossRef CAS PubMed.
- V. G. Stepanov, Y. Xiao, Q. Tran, M. Rojas, R. C. Willson, Y. Fofanov, G. E. Fox and D. J. Roberts, BMC Microbiol., 2014, 14, 225 CrossRef PubMed.
- M. Fahrbach, J. Kuever, R. Meinke, P. Kämpfer and J. Hollender, Int. J. Syst. Evol. Microbiol., 2006, 56, 1547 CrossRef CAS PubMed.
- T. Shintani, W. T. Liu, S. Hanada, Y. Kamagata, S. Miyaoka, T. Suzuki and K. Nakamura, Int. J. Syst. Evol. Microbiol., 2000, 50, 201 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |