Facile synthesis of P2-type Na0.4Mn0.54Co0.46O2 as a high capacity cathode material for sodium-ion batteries†
Xijun Xua,
Shaomin Ji*a,
Ruibo Gaob and
Jun Liu*a
aKey Laboratory of Low Dimensional Materials & Application Technology, Ministry of Education, School of Materials Science and Engineering, Xiangtan University, Xiangtan 411105, China. E-mail: smji@xtu.edu.cn; jliu@xtu.edu.cn
bJinzhou Petrochemical Co., LTD (JZPC), No. 2, Chongqin Road, Guta district, Jinzhou city, 121001, Liaoning province, China
First published on 2nd June 2015
Abstract
Sodium-ion batteries have received great attention because of the abundant sodium resources and low cost. As a typical kind of cathode material for Na-ion batteries, sodium manganese oxides have shown great potential in cathode application due to their high specific capacity and good rate capability. Herein, we successfully synthesized P2-type Na0.4Mn0.54Co0.46O2 nanosheets via a two-step annealing route. The morphology and structural information of the Na0.4Mn0.54Co0.46O2 products were characterized by X-ray diffraction (XRD), transmission electron microscopy (TEM) and high resolution transmission electron microscopy (HRTEM) technologies. The electrochemical performances of Na0.4Mn0.54Co0.46O2 were measured by charge–discharge tests, cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). As the cathode for Na-ion batteries, the layered Na0.4Mn0.54Co0.46O2 nanosheets showed a high second charge capacity of 194 mA h g−1 and delivered a specific capacity of 125 mA h g−1 at a current of 20 mA g−1 after 60 cycles.
Introduction
Over the past few decades, Li-ion batteries have obtained significant attention, due to their importance for future renewable energy and smart grids.1–3 Li-ion batteries have good cycling performance and rate capability, they are suitable for small and lightweight electronic products, but their wide use is hindered by the high cost and limited resources of lithium salts.4–7 In recent years, Na-ion batteries have received extensive concern, owing to the lower cost and more natural abundance of sodium resources.8,9 Furthermore, in contrast to Li-ion batteries which use copper as the anode current collector, Na-ion batteries can use the cheaper and more abundant Al, as it does not alloy with sodium.8 In order to meet the ever-growing market demand for energy storage devices, therefore, much effort has been made to explore Na-ion batteries to partly substitute currently commercialized Li-ion batteries. Therefore, the search for new energetic electrode materials, especially cathode materials, for Na-ion batteries has been highlighted in the battery industry and energy field.10The layered P2-type sodium manganese oxides have been extensively studied as such a kind of promising cathode materials for Na-ion batteries due to their high capacity and low cost.8–12 Unfortunately, the application of the layered manganese-based cathode materials was seriously hindered by several problems: (a) the main issue is the ionic radius of Na+ (0.98 Å) which is greater than that of Li+ (0.68 Å), as a result the stability and diffusion of Na-ion system is worse than that of Li-ion system;13 (b) an inferior work potential and specific energy densities compared to Li-ion batteries;14,15 (c) the irreversible changes and rapid decrease of cathode' capacity originate from the manganese ions dissolve in the electrolyte caused by acid solution and Jahn–Teller effect;16–19 (d) finally, the low specific capacity owing to a large polarization at high current densities, resulting from sluggish Na+ ion diffusion in the layered materials.15 Rational designing electrode materials as substituting a portion of Mn ions with other transition metal ions is an effective way to solve these problems.20–22 As we known, doping a portion of Co ions in NaxMnO2 materials as Na-ion battery cathode can improve the charge–discharge voltage. Introducing ions with lower valance state into Mn sites may increase the valance state of Mn3+ to Mn4+ (ref. 23). These motivated us to substitute a portion of Mn ions with Co ions and a new Co-doped sodium manganese oxides material has been designed for Na-ion batteries.
Many layered cathode materials have been successfully used in Li-ion batteries, such as layered LixMnyO2, LiCoO2, and nickel manganese oxide of LiNi1/2Mn1/2O2.17,24,25 Na-based layered electrode materials can be categorized into two main groups using the classification proposed: O3 type and P2 type, in which the sodium ions are accommodated at octahedral and prismatic sites respectively.26 Till now, significant progress has been made to explore new Na-intercalation compounds for rechargeable Na-ion batteries. P2-type NaxMnO2 and NaxCoO2 have been synthesized as cathode materials with high capacity, good cycle stability and rate capability.11,27–29 For example, Bruce et al. investigated β-NaMnO2, which have a different structure from that of NaMnO2 polymorphs and other compounds. It exhibited a high capacity (190 mA h g−1 at a rate of C/20), along with a good rate capability (142 mA h g−1 at a rate of 2 C) and a good capacity retention (100 mA h g−1 after 100 cycles at a rate of 2 C).30 The available reversible capacity of P2-Nax[Fe1/2Mn1/2]O2 reaches 190 mA h g−1 with an average voltage of 2.75 V versus sodium metal.31 The energy density is estimated to be 520 mW h g−1, which is comparable to that of LiFePO4 (about 530 mW h g−1 versus Li) and slightly higher than that of LiMn2O4 (about 450 mW h g−1).17,31 The above research progress is proved that layered P2-type Co-doped sodium manganese oxides are promising cathode materials for Na-ion batteries. It is well know that reducing the manganese content and raising the average valence of manganese in the layered manganese-based cathode materials are effective ways to alleviate the manganese dissolution and Jahn–Teller effect. As we known, high sodium contents normally result in O3-type oxide cathodes, while low contents for P2-type ones with a higher capacity than O3-type.26,32,33 Herein we have designed a layered Na0.4Mn0.54Co0.46O2 cathode material with low sodium content for superior Na-ion batteries. The P2-Na0.4Mn0.54Co0.46O2 has a good specific capacity and cycling performance at a current of 20 mA g−1, and a specific capacity of 120 mA h g−1 is still achieved after 67 cycles.
Experimental section
Materials synthesis
MnCO3 was synthesized by a precipitation method. In a typical synthesis, 10 mmol Mn(NO3)2 was dissolved in 200 mL distilled water, then 200 mL of 0.5 mol L−1 NH4HCO3 was added into the Mn(NO3)2 solution. Spherical Mn2O3 was synthesized by annealing microsphere MnCO3 at 400 °C for 10 h in air condition. The P2-Na0.4Mn0.54Co0.46O2 cathode was synthesized by mixing 5 mmol Mn2O3, 5 mmol Co(NO3)2, 4 mmol NaOH and 6 mmol NaNO3 into 100 mL ethanol, evaporating the ethanol in water bath at 80 °C. Finally, the obtained powder was heated in air at 5 °C s−1 up to 900 °C and kept at this temperature for 12 h to form P2-Na0.4Mn0.54Co0.46O2 nanosheets.Materials characterization
The collected products were characterized by X-ray diffractometry (XRD) with a Rigaku-DMax 2400 diffractometer equipped with a graphite-monochromated Cu-Kα 426 radiation source at a scanning rate of 0.02° s−1. Scanning electron microscopy (SEM) analysis was performed with JSM-6610LV. The detailed crystal structures were further analyzed by transmission electron microscope (TEM) and high-resolution transmission electron microscope (HRTEM) JEM-2100. The proportion of element was tested by energy dispersive spectrometer (EDS) SUTW-SAPPHIRE.Electrochemical testing
For the preparation of the working electrode, a mixture of Na0.4Mn0.54Co0.46O2, conducting additive (acetylene black), and polyvinylidene fluoride (PVDF) in a weight ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 was ground with N-methy1-2-pyrrolidone (NMP) as solvent to make slurry. The slurry was then applied to an Al foil and dried in vacuum oven at 80 °C for 12 h to from the working electrode. The test cell (CR2025) which were assembled in an Ar-filled glove box (H2O and O2 < 1 ppm). Each cell typically contained 2.0–3.0 mg cm−2 of the active materials. A circular Na foil was used as the counter electrode and celgard2400 as the separator. A solution of 1 mol NaClO4 in ethylene carbonate/diethyl carbonate (EC/DEC = 4
10 was ground with N-methy1-2-pyrrolidone (NMP) as solvent to make slurry. The slurry was then applied to an Al foil and dried in vacuum oven at 80 °C for 12 h to from the working electrode. The test cell (CR2025) which were assembled in an Ar-filled glove box (H2O and O2 < 1 ppm). Each cell typically contained 2.0–3.0 mg cm−2 of the active materials. A circular Na foil was used as the counter electrode and celgard2400 as the separator. A solution of 1 mol NaClO4 in ethylene carbonate/diethyl carbonate (EC/DEC = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 in volume) was used as the electrolyte. Galvanostatic discharge and charge at various current densities were performed on a NEWARE-BTS battery tester, with cut-off potentials of 1.5 V for discharge and 4.2 V for charge. Cyclic voltammetry (CV) was performed on a CHI660D electrochemistry workstation at the scan rate of 0.1 mV s−1.
6 in volume) was used as the electrolyte. Galvanostatic discharge and charge at various current densities were performed on a NEWARE-BTS battery tester, with cut-off potentials of 1.5 V for discharge and 4.2 V for charge. Cyclic voltammetry (CV) was performed on a CHI660D electrochemistry workstation at the scan rate of 0.1 mV s−1.
Results and discussion
Fig. 1 illustrates the detail experiment process of P2-Na0.4Mn0.54Co0.46O2 cathode. The P2-type cathode materials were synthesized through a two-step annealed route. Firstly, Mn(NO3)2 and NH4HCO3 were dissolved in distilled water to form a clear solution respectively, then they were mixed together to form a hybrid solution and the white precipitation appeared immediately. After filtered and dried, the precipitation powder annealed at 400 °C for 10 h to obtain Mn2O3. In the next step, the mixed Mn2O3, Co(NO3)2, NaNO3, NaOH (in a mole ratio 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) powder was poured into ethanol and then evaporated the ethanol in water bath at 80 °C. Finally, the obtained powder was heated at 900 °C for 12 h to form the final product of P2–Na0.4Mn0.54Co0.46O2 nanosheet products. This facile and general route has also been successfully applied to synthesis of a higher cobalt content oxide cathode of Na0.4Co0.6Mn0.4O2.
6) powder was poured into ethanol and then evaporated the ethanol in water bath at 80 °C. Finally, the obtained powder was heated at 900 °C for 12 h to form the final product of P2–Na0.4Mn0.54Co0.46O2 nanosheet products. This facile and general route has also been successfully applied to synthesis of a higher cobalt content oxide cathode of Na0.4Co0.6Mn0.4O2.
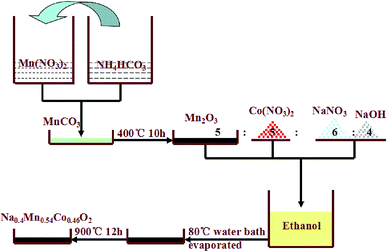 | ||
| Fig. 1 The detailed schematic process of the synthesis of P2-type Na0.4Mn0.54Co0.46O2 nanosheet cathode for Na-ion batteries from Mn2O3 precursor. | ||
The detail structure information of the as-prepared cathode samples was firstly characterized by X-ray diffraction (XRD) technology. As clearly shown in Fig. 2a, the XRD pattern of the obtained P2-Na0.4Mn0.54Co0.46O2 products was well consistent with Na0.45Ni0.22Co0.11Mn0.66O2 and Na0.5[Ni0.23Fe0.13Mn0.63]O2 reported before,32,33 indicating that the as-obtained P2-Na0.4Mn0.54Co0.46O2 is performs a high purity and good crystallinity. The XRD pattern of P2-Na0.4Mn0.54Co0.46O2 can also be ascribed to Na0.7MnO2.05 (JCPDS no. 27-0731, system: hexagonal, a = 2.876 Å, c = 11.12 Å), proving that the final Na0.4Mn0.54Co0.46O2 oxide is a layered structure (space group P63mmc). As shown in Fig. 2b, the transition metal Mn and Co constituted the MeO2 (Me = Mn, Co) layer, the Na+ was insert and extract in a trigonal prismatic which was consist of 6 oxygen atoms. In such structure, the sodium ions occupy the trigonal prismatic sites in one layer, whereas Co and Mn ions co-occupy the neighboring layer.4,34 Na atoms are in the 2b and 2d sites, the Co and Mn atoms are ordered regularly in 2a sites, and O atoms are occupied the 4f sites. The refined lattice parameters (a = 2.831 Å, c = 11.286 Å) of Na0.4Mn0.54Co0.46O2 are a little different with that of Na0.7MnO2.05 (a = 2.876 Å, c = 11.2 Å, Table S1 in ESI†). The little smaller lattice parameter (a = 2.831 Å) of Na0.4Mn0.54Co0.46O2 is caused by the replacement of Jahn–Teller Mn3+ (0.645 Å) with the smaller radius Co3+ (0.545 Å), while the little larger lattice parameter (c = 11.286 Å) is caused by a large repulsive force between the MeO2 layer, which is related to the less content of sodium in the Na0.4Mn0.54Co0.46O2 (ref. 34).
 | ||
| Fig. 2 (a) The typical XRD patterns of Na0.4Mn0.54Co0.46O2 nanosheets (red curve) and JCPDS no. 27-751 (black curve); (b) structural illustration of P2-type Na0.4Mn0.54Co0.46O2. | ||
The microstructure of the simple Mn2O3 precursor and final Na0.4Mn0.54Co0.46O2 cathode products are shown in Fig. 3. Fig. 3a and b are the typical low- and high-magnification SEM images of Mn2O3 precursor, respectively, which illustrate the uniformity of spherical morphology. The pure phase of Mn2O3 precursor can be clearly proved by the corresponding XRD patterns (Fig. S1 in ESI†). The morphology and size of P2-Na0.4Mn0.54Co0.46O2 particles can be also clearly observed from SEM images (Fig. 3c and d), revealing that during the following high-temperature annealing treatment, these simple Mn2O3 precursor microspheres has been totally transformed into complex Na0.4Mn0.54Co0.46O2 nanosheets. The P2-Na0.n0.54Co0.46O2 cathode is consisted of uniform 2D nanosheets with thickness of about 500 nm (Fig. 3d). The detailed crystal structure of Na0.4Mn0.54Co0.46O2 was further analyzed by transmission electron microscope (TEM) and high-resolution TEM (HRTEM). The nanosheet feature of these Na0.4Mn0.54Co0.46O2 cathode particles were strongly confirmed by the low-magnification TEM images (Fig. 4a and b). Fig. 4c is the high-magnification TEM image of Na0.4Mn0.54Co0.46O2, indicating that the Na0.4Mn0.54Co0.46O2 cathode material exhibits a layered nanostructure. Fig. 4d is the typical HRTEM image of the Na0.4Mn0.54Co0.46O2, from which we can directly observe the regular atomic arrangement of Na0.4Mn0.54Co0.46O2, and the lattice spacing of the crystal plane (002) and (004) was 5.64 Å and 2.81 Å, respectively. These results reflect that Na+ can facilely insert and extract at inter-layer of Na0.4Mn0.54Co0.46O2, and also directly reveal that the (001) crystal plane was not the electrochemical active for Na+ insertion and extraction, while the (100) was the exposed crystal plane for Na+ insertion and extraction.27,34
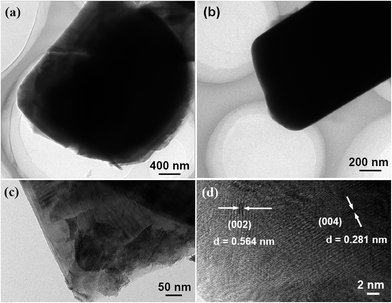 | ||
| Fig. 4 Low- (a and b), high-magnification (c) TEM, and HRTEM (d) images the finally achieved P2-type Na0.4Mn0.54Co0.45O2 nanosheet cathode for Na-ion batteries. | ||
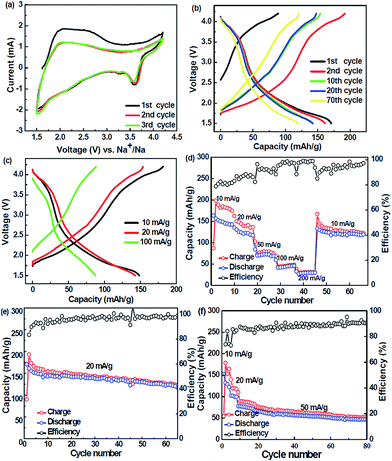
In order to investigate the Na-ion storage properties, the P2-Na0.4Mn0.54Co0.46O2 were used as cathode with the standard Na0.4Mn0.54Co0.46O2/Na half-cell configuration. The electrochemical reactions of layered Na0.4Mn0.54Co0.46O2 as cathode in Na-ion batteries were firstly investigated by cyclic voltammetry (CV) measurements, as presented in Fig. 5a. The CV curves are well-overlapped for the initial three tested cycles, revealing a high reversibility of the P2-Na0.4Mn0.54Co0.46O2 nanosheets. It can be founded that there is irreversible capacity in the first cycle. The irreversible capacity may originate from the reaction between the cathode and electrolyte, due to the high cut-off voltages of 4.2 V.37 In these CV curves, the peaks of Na0.4Mn0.54Co0.46O2 were shown at 1.5–3.0 V, which relates to the redox reactions of the Mn4+/Mn3+ and Co3+/Co2+ ionic pairs. The peaks at 3.0–4.2 V are assumed to be associated with the redox reactions of the Co4+/Co3+ ionic pair.35–40 The peak around 4.1 V is related to the MeO2 sheet shift, which is take place at low sodium contents, reducing the repulsion between oxygen atoms in the trigonal prismatic structure.35 Along with the charging process, this material takes a phase transition from P2 phase to OP4 phase; in the discharge process, it takes from OP4 phase to P2 phase.26 The integrated area of low voltage region is almost equaled with the integrated area of high voltage region, revealing the similar content of cobalt and manganese elements in the Na0.4Mn0.54Co0.46O2 cathode.
Owing to the decomposition of electrolyte and form the SEI membrane, the P2-Na0.4Mn0.54Co0.46O2 cathode delivered a charge capacity larger than the discharge capacity in the initial cycle (Fig. 5b). With the charge/discharge cycles increased, the decay of capacity became slow, and the coulombic efficiency increased. The P2-Na0.4Mn0.54Co0.46O2 electrode presented a superior cycle performance and good capacity retention. It can deliver a specific capacity of about 120 mA h g−1 at 20 mA g−1 after 67 cycles (Fig. 5e). The excellent cycling stability for the P2-Na0.4Mn0.54Co0.46O2 electrode can be explained by two factors: the introduction of Co with a low valance can elevate the average valance of Mn to alleviate the Jahn–Teller distortion; the better electrochemical reversibility of Co as compared Mn can decrease the polarization during sodium insertion and extraction to suppress the structural stress of the materials.38 The charge/discharge curves at different current densities (10 mA g−1, 20 mA g−1, and 100 mA g−1) are shown in Fig. 5c. The Na0.4Mn0.54Co0.46O2 nanosheets delivered a charge capacity of 184 mA h g−1, 153 mA h g−1 and 90 mA h g−1 at current density of 10 mA g−1, 20 mA g−1 and 100 mA g−1, respectively. The low specific capacity owing to a large polarization at high current densities resulted from sluggish Na+ ion diffusion in the layered materials.15 The charge–discharge capacity of Na0.4Mn0.54Co0.46O2 nanosheets at different current densities (10 mA g−1, 20 mA g−1, 50 mA g−1, 100 mA g−1, and 200 mA g−1) are shown in Fig. 5d. The Na0.4Mn0.54Co0.46O2 exhibits a high charge capacity of 180 mA h g−1, but relatively low coulombic efficiency at 10 mA g−1, indicating the irreversible capacity in Na0.4Mn0.54Co0.46O2 cathode. The irreversible capacity originated from the reaction between the cathode and electrolyte as the high cut-off voltage of 4.2 V, which is consistent with the CV result (Fig. 5a).34 In addition, the charge capacity of Na0.4Mn0.54Co0.46O2 is higher than its discharge capacity, which enables to combine a Na0.4Mn0.54Co0.46O2 cathode with any kind of carbonaceous or metallic anode to construct sodium based full-cell system.15 It shows a charge and discharge capacity of 145 mA h g−1, 128 mA h g−1 at 20 mA g−1, respectively, with a coulombic efficiency of 88% (Fig. 5d). When the charge–discharge current density increased to 50 mA g−1, the charge and discharge capacity decreased to 75 mA h g−1 and 68 mA h g−1, respectively, and the corresponding coulombic efficiency is 90%. Furthermore, as the current density further increased to 100 mA g−1 and 200 mA g−1, the specific capacity continues reduced, but the coulombic efficiency gradually increased to about 100%. The large polarization at a high current density can result a low specific capacity. It can be observed that when the current density turned back to 10 mA g−1, the cell capacity recovered to 150 mA h g−1, indicating good retention after high-rate cycling. The P2-Na0.4Mn0.54Co0.45O2 shows a good cycling performance and capacity retention at a charge–discharge current density of 20 mA g−1. After 65 cycles, the P2-Na0.4Mn0.54Co0.46O2 nanosheets can still deliver a specific capacity of 125 mA h g−1, with a coulombic efficiency of 95% and a capacity retention of 65%. Such electrochemical performance of P2-Na0.4Mn0.54Co0.46O2 nanosheet cathode make it likely application in rechargeable Na-ion batteries. As shown in Fig. 5d, there is one cycle with coulombic efficiency over 100%, which may caused by the testing temperature and other factors. From the initial and second charge specific capacity, it can roughly calculate the sodium content in the P2-Na0.4Mn0.54Co0.46O2 cathode materials is about 45%, which is close to the energy dispersive spectrometer (EDS) results (Fig. S2 in ESI†). Fig. 5f shows the cycling stability of Na0.4Mn0.54Co0.46O2 nanosheets cathode at a current of 50 mA g−1. As shown in this figure, it can be clearly observed that a fairly stable charge–discharge capacity after 80 cycles was achieved. The low charge–discharge capacity at a large current density can be attributed to the materials irreversible change caused by Jahn–Teller effect and Mn2+ ions dissolution. The large polarization at a high current density can also result a low specific capacity. As the valance of Mn and Co in the materials is +4 and +3 (ref. 26, 38 and 39), substitute Co3+ with such a high content can improve all Mn ion from +3 to +4 valance, which can greatly reduce the Jahn–Teller effect and achieve a superior cycle performance. Another oxide cathode with different ratio of Mn/Co (P2-Na0.4Mn0.4Co0.6O2) was also synthesized by the current two-step annealing route. As shown in Fig. S4 (see in ESI†), the P2-Na0.4Mn0.4Co0.6O2 cathode can display a stable capacity of 130 mA h g−1 at 10 mA g−1.
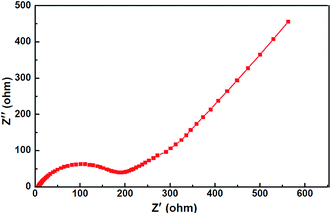
To further understand the electrochemical performance of Na0.4Mn0.54Co0.46O2 nanosheets, electrochemical impedance spectrum (EIS) were performed. Generally, the EIS has been proved to be useful and important tools for analyzing the dynamics of sodium insert and extract electrodes.40–42 The Nyquist plots of the Na0.4Mn0.54Co0.46O2 were obtained in the frequency range from 100 kHz to 0.1 Hz (Fig. 6). The EIS clearly show a semicircle in high-frequency range, which is related to the diffusion resistance of Na+ through the SEI membrane; and the semicircle in medium-frequency range is associated with the charge transfer resistance between the Na0.4Mn0.54Co0.46O2 cathode materials and the liquid electrolyte.42–44 The inclined line in low-frequency region reflects the solid state diffusion of Na+ in the active materials.40 From the impedance spectra of Na0.4Mn0.54Co0.46O2 can observed that the new active cathode materials shows a low resistance, which is consistent with its good cycling performance and capacity retention rate.
Conclusion
In summary, we have successfully synthesized the layered structure cathode of Na0.4Mn0.54Co0.46O2 nanosheets. The obtained cathode materials show a high specific capacity and superior cycling performance, and can potentially be applied in secondary Na-ion batteries. The P2-Na0.4Mn0.54Co0.46O2 nanosheets exhibited a high reversible capacity of 151 mA h g−1 at a current of 20 mA g−1. After 65 charging/discharging cycles, they still delivered a reversible capacity of 120 mA h g−1 at 20 mA g−1. Such high capacity and superior cycling performance promise these P2-Na0.4Mn0.54Co0.46O2 nanosheets obtained via such a simple precipitation and annealing treatment a bright future in rechargeable Na-ion batteries.Acknowledgements
This work was financially supported by National Natural Science Foundation of China (11202177, 51202207).References
- Z. Wang and L. Zhou, Metal Oxide Hollow Nanostructures for Lithium-Ion Batteries, Adv. Mater., 2012, 24(14), 1903–1911 CrossRef CAS PubMed.
- B. Qu, M. Zhang, D. Lei, Y. Zeng, Y. Chen, L. Chen, Q. Li, Y. Wang and T. Wang, Facile solvothermal synthesis of mesoporous Cu2SnS3 spheres and their application in lithium-ion batteries, Nanoscale, 2011, 3(9), 3646–3651 RSC.
- J. Liu, Y. Zhou, C. Liu, J. Wang, Y. Pan and D. Xue, Self-assembled porous hierarchical-like CoO@C microsheets transformed from inorganic–organic precursors and their lithium-ion battery application, CrystEngComm, 2012, 14(8), 2669–2674 RSC.
- J.-M. Tarascon, Is lithium the new gold?, Nat. Chem., 2010, 2(6), 510 CrossRef CAS PubMed.
- L. Zhang, J. Deng, L. Liu, W. Si, S. Oswald, L. Xi, M. Kundu, G. Ma, T. Gemming, S. Baunack, F. Ding, C. Yan and O. G. Schmidt, Hierarchically designed SiOx/SiOy bilayer nanomembranes as stable anodes for lithium ion batteries, Adv. Mater., 2014, 26(26), 4527–4532 CrossRef CAS PubMed.
- W. Si, I. Mönch, C. Yan, J. Deng, S. Li, G. Lin, L. Han, Y. Mei and O. G. Schmidt, A Single Rolled-Up Si Tube Battery for the Study of Electrochemical Kinetics, Electrical Conductivity, and Structural Integrity, Adv. Mater., 2014, 26(47), 7973–7978 CrossRef CAS PubMed.
- J. Deng, H. Ji, C. Yan, J. Zhang, W. Si, S. Baunack, S. Oswald, Y. Mei and O. G. Schmidt, Naturally Rolled-Up C/Si/C Trilayer Nanomembranes as Stable Anodes for Lithium-Ion Batteries with Remarkable Cycling Performance, Angew. Chem., Int. Ed., 2013, 125(8), 2382–2386 CrossRef PubMed.
- N. Bucher, S. Hartung, A. Nagasubramanian, Y. L. Cheah, H. E. Hoster and S. Madhavi, Layered NaxMnO2+z in Sodium Ion Batteries-Influence of Morphology on Cycle Performance, ACS Appl. Mater. Interfaces, 2014, 6(11), 8059–8065 CAS.
- J. Liu, Y. Wan, W. Liu, J. Wang, Y. Zhou and D. Xue, Advanced Nanostructured Cathode and Anode Materials for High-Performance Li-Ion Batteries, Energy Environ. Focus, 2012, 1(1), 19–38 CrossRef PubMed.
- T. Shibata, W. Kobayashi and Y. Moritomo, Sodium ion diffusion in layered NaxMnO2 (0.49 ≤ x ≤ 0.75): Comparison with NaxCoO2, Appl. Phys. Express, 2014, 7(6), 067101 CrossRef.
- Y. Mo, S. P. Ong and G. Ceder, Insights into Diffusion Mechanisms in P2 Layered Oxide Materials by First-Principles Calculations, Chem. Mater., 2014, 26(18), 5208–5214 CrossRef CAS.
- T. Shimono, D. Tanabe, W. Kobayashi, H. Nitani and Y. Moritomo, Electronic State of P2-Type NaxMO2 (M = Mn and Co) as Investigated by In situ X-ray Absorption Spectroscopy, J. Phys. Soc. Jpn., 2013, 82, 124717–124722 CrossRef.
- M. Xie, R. Luo, J. Lu, R. Chen, F. Wu, X. Wang, C. Zhan, H. Wu, H. M. Albishri and A. S. Al-Bogami, Synthesis-microstructure-performance relationship of layered transition metal oxides as cathode for rechargeable sodium batteries prepared by high-temperature calcination, ACS Appl. Mater. Interfaces, 2014, 6(19), 17176–17183 CAS.
- S. P. Ong, V. L. Chevrier, G. Hautier, A. Jain, C. Moore, S. Kim, X. Ma and G. Ceder, Voltage, stability and diffusion barrier differences between sodium-ion and lithium-ion intercalation materials, Energy Environ. Sci., 2011, 4(9), 3680–3688 CAS.
- D. W. Han, J. H. Ku, R. H. Kim, D. J. Yun, S. S. Lee and S. G. Doo, Aluminum Manganese Oxides with Mixed Crystal Structure: High-Energy-Density Cathodes for Rechargeable Sodium Batteries, ChemSusChem, 2014, 7(7), 1870–1875 CrossRef CAS PubMed.
- W. Liu, J. Liu, K. Chen, S. Ji, Y. Wan, Y. Zhou, D. Xue, P. Hodgson and Y. Li, Enhancing the Electrochemical Performance of the LiMn2O4 Hollow Microsphere Cathode with a LiNi0.5Mn1.5O4 Coated Layer, Chem.–Eur. J., 2014, 20(3), 824–830 CrossRef CAS PubMed.
- L. Zhou, D. Zhao and X. D. Lou, LiNi0.5Mn1.5O4 Hollow Structures as High-Performance Cathodes for Lithium-Ion Batteries, Angew. Chem., Int. Ed., 2012, 124(1), 243–245 CrossRef PubMed.
- X. Li, X. Ma, D. Su, L. Liu, R. Chisnell, S. P. Ong, H. Chen, A. Toumar, J.-C. Idrobo and Y. Lei, Direct visualization of the Jahn–Teller effect coupled to Na ordering in Na5/8MnO2, Nat. Mater., 2014, 13, 586–592 CrossRef CAS PubMed.
- N. Van Nghia, P.-W. Ou and I.-M. Hung, Synthesis and Electrochemical Properties of Sodium Manganese-based Oxide Cathode Material for Sodium-Ion Batteries, Electrochim. Acta, 2015, 161, 63–71 CrossRef CAS PubMed.
- S. Komaba, N. Yabuuchi, T. Nakayama, A. Ogata, T. Ishikawa and I. Nakai, Study on the Reversible Electrode Reaction of Na1–xNi0.5Mn0.5O2 for a Rechargeable Sodium-Ion Battery, Inorg. Chem., 2012, 51(11), 6211–6220 CrossRef CAS PubMed.
- H. Kim, D. J. Kim, D.-H. Seo, M. S. Yeom, K. Kang, D. K. Kim and Y. Jung, Ab initio study of the sodium intercalation and intermediate phases in Na0.44MnO2 for sodium-ion battery, Chem. Mater., 2012, 24(6), 1205–1211 CrossRef CAS.
- B. Mortemard de Boisse, D. Carlier, M. Guignard, L. Bourgeois and C. Delmas, P2-NaxMn1/2Fe1/2O2 Phase Used as Positive Electrode in Na Batteries: Structural Changes Induced by the Electrochemical (De) Intercalation Process, Inorg. Chem., 2014, 53(20), 11197–11205 CrossRef CAS PubMed.
- Y. Wang, X. Yu, S. Xu, J. Bai, R. Xiao, Y.-S. Hu, H. Li, X.-Q. Yang, L. Chen and X. Huang, A zero-strain layered metal oxide as the negative electrode for long-life sodium-ion batteries, Nat. Commun., 2013, 4, 2365 Search PubMed.
- A. R. Armstrong, A. J. Paterson, A. D. Robertson and P. G. Bruce, Nonstoichiometric Layered LixMnyO2 with a High Capacity for Lithium Intercalation/Deintercalation, Chem. Mater., 2002, 14(2), 710–719 CrossRef CAS.
- T. Ohzuku and Y. Makimura, Layered Lithium Insertion Material of LiNi1/2Mn1/2O2: A Possible Alternative to LiCoO2 for Advanced Lithium-Ion Batteries, Chem. Lett., 2001,(8), 744–745 CrossRef CAS.
- (a) N. Yabuuchi, M. Kajiyama, J. Iwatate, H. Nishikawa, S. Hitomi, R. Okuyama, R. Usui, Y. Yamada and S. Komaba, P2-type Nax[Fe1/2Mn1/2]O2 made from earth-abundant elements for rechargeable Na batteries, Nat. Mater., 2012, 11(6), 512–517 CrossRef CAS PubMed; (b) B. E. Douglas and S.-M. Ho, Structures Involving P and O Layers, Struct. Chem. Crystal. Solids, 2006, 63–116 Search PubMed.
- F. Sauvage, L. Laffont, J.-M. Tarascon and E. Baudrin, Study of the insertion/deinsertion mechanism of sodium into Na0.44MnO2, Inorg. Chem., 2007, 46(8), 3289–3294 CrossRef CAS PubMed.
- A. K. Rai, L. T. Anh, J. Gim, V. Mathew and J. Kim, Electrochemical properties of NaxCoO2 (x ∼ 0.71) cathode for rechargeable sodium-ion batteries, Ceram. Int., 2014, 40(1), 2411–2417 CrossRef CAS PubMed.
- S. C. Han, H. Lim, J. Jeong, D. Ahn, W. B. Park, K.-S. Sohn and M. Pyo, Ca-doped NaxCoO2 for improved cyclability in sodium ion batteries, J. Power Sources, 2015, 277, 9–16 CrossRef CAS PubMed.
- J. Billaud, R. J. Clément, A. R. Armstrong, J. Canales-Vázquez, P. Rozier, C. P. Grey and P. G. Bruce, β-NaMnO2: a High Performance Cathode for Sodium-Ion Batteries, J. Am. Chem. Soc., 2014, 136(49), 17243–17248 CrossRef CAS PubMed.
- J. Liu, W. Liu, S. Ji, Y. Zhou, P. Hodgson and Y. Li, Electrospun Spinel LiNi0.5Mn1.5O4 Hierarchical Nanofibers as 5 V Cathode Materials for Lithium-Ion Batteries, ChemPlusChem, 2013, 78(7), 636–641 CrossRef CAS PubMed.
- D. Buchholz, A. Moretti, R. Kloepsch, S. Nowak, V. Siozios, M. Winter and S. Passerini, Toward Na-Ion Batteries Synthesis and Characterization of a Novel High Capacity Na Ion Intercalation Material, Chem. Mater., 2013, 25(2), 142–148 CrossRef CAS.
- I. Hasa, D. Buchholz, S. Passerini, B. Scrosati and J. Hassoun, High Performance Na0.5[Ni0.23Fe0.13Mn0.63]O2 Cathode for Sodium-Ion Batteries, Adv. Energy Mater., 2014, 4(15), 1400083 Search PubMed.
- N. Yabuuchi, R. Hara, M. Kajiyama, K. Kubota, T. Ishigaki, A. Hoshikawa and S. Komaba, New O2/P2-type Li-Excess Layered Manganese Oxides as Promising Multi-Functional Electrode Materials for Rechargeable Li/Na Batteries, Adv. Energy Mater., 2014, 4(13), 1301453 Search PubMed.
- D. Yuan, X. Hu, J. Qian, F. Pei, F. Wu, R. Mao, X. Ai, H. Yang and Y. Cao, P2-type Na0.67Mn0.65Fe0.2Ni0.15O2 Cathode Material with High-capacity for Sodium-Ion Battery, Electrochim. Acta, 2014, 116, 300–305 CrossRef CAS PubMed.
- Y. Wen, B. Wang, G. Zeng, K. Nogita, D. Ye and L. Wang, Electrochemical and Structural Study of Layered P2-Type Na2/3Ni1/3Mn2/3O2 as Cathode Material for Sodium-Ion Battery, Chem.–Asian J., 2015, 10(3), 661–666 CrossRef CAS PubMed.
- D. Carlier, J. Cheng, R. Berthelot, M. Guignard, M. Yoncheva, R. Stoyanova, B. Hwang and C. Delmas, The P2-Na2/3Co2/3Mn1/3O2 phase: structure, physical properties and electrochemical behavior as positive electrode in sodium battery, Dalton Trans., 2011, 40(36), 9306–9312 RSC.
- X. Wang, M. Tamaru, M. Okubo and A. Yamada, Electrode Properties of P2-Na2/3MnyCo1–yO2 as Cathode Materials for Sodium-Ion Batteries, J. Phys. Chem. C, 2013, 117(30), 15545–15551 CAS.
- J.-H. Cheng, C.-J. Pan, J.-F. Lee, J.-M. Chen, M. Guignard, C. Delmas, D. Carlier and B.-J. Hwang, Simultaneous Reduction of Co3+ and Mn4+ in P2-Na2/3Co2/3Mn1/3O2 As Evidenced by X-ray Absorption Spectroscopy during Electrochemical Sodium Intercalation, Chem. Mater., 2014, 26(2), 1219–1225 CrossRef CAS.
- H. Ma, S. Zhang, W. Ji, Z. Tao and J. Chen, α-CuV2O6 nanowires: Hydrothermal synthesis and primary lithium battery application, J. Am. Chem. Soc., 2008, 130(15), 5361–5367 CrossRef CAS PubMed.
- D. Aurbach, M. Levi, K. Gamulski, B. Markovsky, G. Salitra, E. Levi, U. Heider, L. Heider and R. Oesten, Capacity fading of LixMn2O4 spinel electrodes studied by XRD and electroanalytical techniques, J. Power Sources, 1999, 81, 472–479 CrossRef.
- J. Liu, W. Liu, S. Ji, Y. Wan, H. Yin and Y. Zhou, Facile Synthesis of Carbon-Encapsulated Li4Ti5O12@C Hollow Microspheres as Superior Anode Materials for Li-Ion Batteries, Eur. J. Inorg. Chem., 2014, 2014(12), 2073–2079 CrossRef CAS PubMed.
- J. Li, S. Xiong, Y. Liu, Z. Ju and Y. Qian, Uniform LiNi1/3Co1/3Mn1/3O2 hollow microspheres: Designed synthesis, topotactical structural transformation and their enhanced electrochemical performance, Nano Energy, 2013, 2(6), 1249–1260 CrossRef CAS PubMed.
- J. Liu, W. Liu, S. Ji, Y. Wan, M. Gu, H. Yin and Y. Zhou, Iron Fluoride Hollow Porous Microspheres: Facile Solution-Phase Synthesis and Their Application for Li-Ion Battery Cathodes, Chem.–Eur. J., 2014, 20(19), 5815–5820 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra06275f |
| This journal is © The Royal Society of Chemistry 2015 |