Surface modification of a LiNi0.5Mn1.5O4 cathode with lithium boron oxide glass for lithium-ion batteries
Chenqiang Dua,
Man Yang*a,
Jie Liua,
Shuting Suna,
Zhiyuan Tang*a,
Deyang Qub and
Xinhe Zhangb
aDepartment of Applied Chemistry, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China. E-mail: 369469876@qq.com; Fax: +86-769-83195372; Tel: +86-769-83017180
bMcNair Technology Company Limited, Dongguan City, Guangdong 523700, P. R. China
First published on 23rd June 2015
Abstract
Lithium boron oxide glass (LBO-glass) coated LiNi0.5Mn1.5O4 cathode materials have been synthesized by a solution method to enhance the electrochemical performances. The structure and morphology of the as-prepared materials have been characterized by XRD, SEM, and TEM. The results indicate that the LiNi0.5Mn1.5O4 is coated with a layer of amorphous LBO-glass. The electrochemical properties are characterized by galvanostatic charge–discharge cycling, cyclic voltammetry, electrochemical impedance spectroscopy and a self-discharge test. Differential scanning calorimetry is carried out to confirm the improved safety by LBO-glass coating. The LBO coating can effectively enhance the electrochemical kinetics of the LiNi0.5Mn1.5O4 phase and improve the cycling performance. Among the as-prepared samples, the 1 wt% LBO-glass coated LiNi0.5Mn1.5O4 presents optimal electrochemical behaviors with a capacity retention of 91.4% after 100 cycles at 1 C and a discharge capacity of 105.8 mA h g−1 at 10 C. Besides, the electrochemical impedance spectroscopy analysis shows the 1 wt% LBO-glass coating reduces the electrochemical impedance and improves the ability to conduct Li+ in the cells to a great extent.
1. Introduction
Lithium-ion batteries have been successful for portable electronic devices such as cell phones and laptop computers due to their higher energy density compared to other rechargeable systems. Aggressive research has recently been focused on the development and optimization of advanced cathode materials for electric vehicles (EV) and hybrid electric vehicles (HEV). However, the existing battery technology established for portable electronics has faced great challenges to meet the criteria of automotive applications, especially in terms of energy density. One effective strategy to increase energy density is to increase the operating voltage of the battery.1 A series of high-voltage cathode materials (LiMxMn2−xO4, M = Ni, Co, Cr, Cu, Fe) have been investigated in the pursuit of substituting transition metals (M) for the Mn site in LiMn2O4,2 among which LiNi0.5Mn1.5O4 has drawn much attention because of its high specific capacity (147 mA h g−1) and high operating voltage (4.7 V vs. Li+/Li).However, the poor electrochemical performances of LiNi0.5Mn1.5O4 such as low discharge capacity and depressed cyclability hinder its application to a large extent.3 One reason is the decomposition of electrolyte at high operating voltage,4,5 resulting in the formation of SEI film, which blocks the insertion/extraction of Li+ and hinders the charge transfer, hence the kinetics of the electrochemical processes. Besides, side reactions between the cathode material and the electrolyte restrict its electrochemical performance.6 Also, the minor Mn3+ ions in LiNi0.5Mn1.5O4, which result from high-temperature and long-time calcinations, further lead to Mn dissolution, structure distortion, and capacity fading upon cycling.7 To overcome these disadvantages, many efforts have been made, such as ion doping8–20 and surface coating.21–29 Cation and/or anion doping such as Zn2+,8 Mg2+,9,10 Al3+,11,12 Fe3+,13 Cr3+,11,14,15 Sm3+,16 Ru4+,17,18 Ti4+,18 Nb5+,19 W6+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and F−
20 and F−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 have been employed to improve the electrochemical behaviours of LiNi0.5Mn1.5O4. However, doping is not able to prevent the decomposition of the electrolyte and side reactions between cathode and electrolyte. In recent years, various oxides and phosphates have drawn researchers' attention because of facile synthesis methods and positive effects on the electrochemical properties of LiNi0.5Mn1.5O4, such as ZnO,21,22 ZrO2,23 ZrP2O7,23 Al2O3,6,22 ZnAl2O4,24 Bi2O3,6,22 SiO2,25 CuO,26 YBaCu3O7,27 BiOF,28 AlPO4
11 have been employed to improve the electrochemical behaviours of LiNi0.5Mn1.5O4. However, doping is not able to prevent the decomposition of the electrolyte and side reactions between cathode and electrolyte. In recent years, various oxides and phosphates have drawn researchers' attention because of facile synthesis methods and positive effects on the electrochemical properties of LiNi0.5Mn1.5O4, such as ZnO,21,22 ZrO2,23 ZrP2O7,23 Al2O3,6,22 ZnAl2O4,24 Bi2O3,6,22 SiO2,25 CuO,26 YBaCu3O7,27 BiOF,28 AlPO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 and FePO4.29 These previous literature has reported that the surface-modifications using inorganic materials are effective to enhance the electrochemical performances and the thermal stability of the high-voltage LiNi0.5Mn1.5O4 cathode system.
22 and FePO4.29 These previous literature has reported that the surface-modifications using inorganic materials are effective to enhance the electrochemical performances and the thermal stability of the high-voltage LiNi0.5Mn1.5O4 cathode system.
Nevertheless, these coating materials can form barriers to the movement of lithium ions due to low ion conductivity. According to Amatucci and co-workers' report,30 lithium boron oxide glass (LBO-glass) is particularly suitable for surface modification for the following reasons. First of all, molten LBO compositions exhibit good wetting properties with respect to ceramics. Not only the good wetting properties but also the relatively low viscosity of LBO-glass in the molten state allow easy processing and result in even coverage with the use of a minimal amount of material. Secondly, LBO compositions have already been investigated as solid lithium ion conductors which show good ability to conduct Li+.31,32 Furthermore, electrochemical studies have shown that LBO coating is helpful for the 4 V positive electrode materials used in lithium-ion batteries such as LiNi0.8Co0.2O2,33 LiNi1/3Co1/3Mn1/3O2,34 LiMn2O4,35,36 Li1+xMn2O4.37
In this paper, a surface modification method is conducted through coating LBO-glass on LiNi0.5Mn1.5O4 in order to enhance its electrochemical performances. The results of various structure and electrochemical analyses show that the LBO-glass plays a protecting role on the surface of LiNi0.5Mn1.5O4 cathode, thus improving the electrochemical behaviours of the cathode materials.
2. Experimental
2.1. Preparation of cathode materials
For the synthesis of LiNi0.5Mn1.5O4 powders, a co-precipitation method was employed. Stoichiometric amounts of Mn(CH3COO)2·4H2O (A.R., Guangdong Guanghua Sci-Tech Co., Ltd.) and Ni(CH3COO)2·4H2O (A.R., Guangdong Guanghua Sci-Tech Co., Ltd) were dissolved in deionized water, then oxalic acid solution containing 10% excessive H2C2O4·2H2O (A.R., Guangdong Guanghua Sci-Tech Co., Ltd) was dropped slowly into the metal acetate solution under stirring. After being stirred for 6 h, the precipitation was washed, centrifuged and then dried at 80 °C. The dried binary metal oxalate were mixed with stoichiometric Li2CO3 (A.R., Guangdong Guanghua Sci-Tech Co., Ltd) (5% excessive to compensate for the lithium evaporation during high temperature calcination), preheated at 450 °C for 5 h, and then calcined at 800 °C for 12 h with a heating rate of 3 °C min−1. The powder was grinded and denoted as LNMO.The LBO-glass coated LiNi0.5Mn1.5O4 was prepared using a solution method. LiOH·H2O (A.R., Guangdong Guanghua Sci-Tech Co., Ltd) and H3BO3 (A.R., Guangdong Guanghua Sci-Tech Co., Ltd) were mixed in ethanol (A.R., Guangdong Guanghua Sci-Tech Co., Ltd) with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Then bare LiNi0.5Mn1.5O4 was added into the solution, and the mixture was stirred and heated at 80 °C until the solvent was completely evaporated. The powders were grinded, pressed into pellets and heated at 500 °C for 10 h. The melting points of H3BO3 and LiOH·H2O were 186 °C and 445 °C, respectively. Therefore, during heating the liquid H3BO3 and LiOH·H2O could wet the surface of LiNi0.5Mn1.5O4 and reacted with each other, producing the LBO-glass. After the mixture cooled to room temperature, the solid Li2O–2B2O3 glass modified LiNi0.5Mn1.5O4 powders were obtained. The weight ratios of the Li2O–2B2O3 glass to the LiNi0.5Mn1.5O4 powders in the modified samples were 0.5%, 1%, 2% and 3%, which were denoted as 0.005LBO–LNMO, 0.01LBO–LNMO, 0.02LBO–LNMO and 0.03LBO–LNMO, respectively.
2. Then bare LiNi0.5Mn1.5O4 was added into the solution, and the mixture was stirred and heated at 80 °C until the solvent was completely evaporated. The powders were grinded, pressed into pellets and heated at 500 °C for 10 h. The melting points of H3BO3 and LiOH·H2O were 186 °C and 445 °C, respectively. Therefore, during heating the liquid H3BO3 and LiOH·H2O could wet the surface of LiNi0.5Mn1.5O4 and reacted with each other, producing the LBO-glass. After the mixture cooled to room temperature, the solid Li2O–2B2O3 glass modified LiNi0.5Mn1.5O4 powders were obtained. The weight ratios of the Li2O–2B2O3 glass to the LiNi0.5Mn1.5O4 powders in the modified samples were 0.5%, 1%, 2% and 3%, which were denoted as 0.005LBO–LNMO, 0.01LBO–LNMO, 0.02LBO–LNMO and 0.03LBO–LNMO, respectively.
2.2. Physical characterization
The crystal structure of samples was identified by X-ray diffraction (XRD, Rigaku RINT2000) using Cu Kα radiation operated at 30 kV and 20 mA with a scanning speed of 4° min−1, from 10° to 80°. The particle morphologies were observed using scanning electron microscope (SEM, Hitachi, S4800). The high resolution transmission electron microscope (HRTEM, JEM-2100F) was employed to observe the LBO-glass layer on the LiNi0.5Mn1.5O4 material.The thermal stability of the LNMO and 0.01LBO–LNMO was studied using differential scanning calorimetry (DSC-Perkin-Elmer Pyris 1 differential scanning calorimeter). After 2 charge–discharge cycles at 0.1 C rate, the cells were fully-charged and subsequently subjected to 1 h of trickle charge at constant voltage of 5.0 V to ensure the cathode material is completely delithiated. Then, the cells were introduced into a dry glove box and opened carefully and the cathode materials were recovered. The electrodes were slightly dried and then scratched to obtain the cathode material. 5–6 mg materials from each kind of electrodes were packed into small stainless steel DSC capsules and then hermetically sealed. The samples were scanned in the DSC equipment under nitrogen purging from room temperature to 400 °C at a heating rate of 10 °C min−1.
2.3. Electrochemical measurements
Electrochemical evaluations of materials were performed with CR2032 coin cells. The positive electrode was prepared by mixing the cathode materials, Super-P, and polyvinylidene fluoride (PVDF) binder with weight proportion of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in N-methyl-2-pyrrolidone (NMP) to form slurry. Then the slurry was coated onto an aluminium foil, which severed as a current collector, followed by drying at 120 °C for 12 h in a vacuum oven. Finally, the cathode material coated foils were pressed under 10 MPa for 1 min, with a final active material loading about 2.8–3.0 mg cm−2. The cells were fabricated in a vacuum glove box filled with argon, with lithium plate as the negative electrode, polypropylene (Celgard®2300, Celgard Inc. USA) as separator, and 1 M LiPF6 dissolved in ethylene carbonate (EC)–diethyl carbonate (DEC) (1
1 in N-methyl-2-pyrrolidone (NMP) to form slurry. Then the slurry was coated onto an aluminium foil, which severed as a current collector, followed by drying at 120 °C for 12 h in a vacuum oven. Finally, the cathode material coated foils were pressed under 10 MPa for 1 min, with a final active material loading about 2.8–3.0 mg cm−2. The cells were fabricated in a vacuum glove box filled with argon, with lithium plate as the negative electrode, polypropylene (Celgard®2300, Celgard Inc. USA) as separator, and 1 M LiPF6 dissolved in ethylene carbonate (EC)–diethyl carbonate (DEC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, by volume ratio) as electrolyte.
1, by volume ratio) as electrolyte.
The charge–discharge tests were performed in the voltage range of 3.5–5.0 V (vs. Li+/Li) on an automatic batteries tester (Land CT 2001A, Wuhan, China). The cycling stability was measured at 1 C (1 C = 147 mA h g−1) for 100 cycles. And to test the rate performance of the samples, the cells were charged at 0.5 C and discharged at different rates. Cyclic voltammetry (CV) tests were conducted on an electrochemical workstation (CHI1040B, ChenHua, China) with a voltage range of 3.5–5.0 V (vs. Li+/Li) at a scanning rate of 0.1 mV s−1. The electrochemical impedance spectroscopy (EIS) analysis of LNMO and 0.01LBO–LNMO was carried out after the cells were charged and discharged at 1 C for 1 cycle and 30 cycles respectively, on an electrochemical workstation (Gamry PC14-750) in the frequency range of 100 kHz to 10 mHz. Self-discharge of LNMO and 0.01LBO–LNMO was investigated on an electrochemical workstation (CHI1040B, ChenHua, China). The cells made by LNMO and 0.01LBO–LNMO were charged and discharged at 0.1 C (1 C = 147 mA h g−1) in the voltage range of 3.5–5.0 V for 5 cycles, then charged to 5 V and left at open-circuit voltage for 10 days. All electrochemical measurements have been performed at room temperature.
3. Results and discussion
3.1. Physical characterization
The XRD patterns of pristine and LBO-glass coated LiNi0.5Mn1.5O4 are shown in Fig. 1. All the samples show sharp peaks of similar location and intensity, and these peaks can be well indexed to the spinel structured LNMO (JCPDS card no. 80-2162, Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase). It can be seen that there are weak peaks at 37.5°, 43.6° and 63.3° in the patterns of samples attributed to the secondary phases LixNi1−xO, which is a common phenomenon in the preparation of LiNi0.5Mn1.5O4. As previous literature reports,38–40 when the annealing temperature is above 650 °C, LiNi0.5Mn1.5O4 phase suffers a decomposition reaction as shown in eqn (1), while the LixNi1−xO phase has no negative effects on the electrochemical performance of LiNi0.5Mn1.5O4.41 As a result, oxygen deficiency occurs and to maintain charge balance, some Mn3+ ions appear instead of Mn4+ ions, which is helpful to improve the electronic conductivity of LiNi0.5Mn1.5O4.38 The peaks of LBO cannot be detected, which is probably due to the low proportion of the LBO-glass or its amorphous nature.33
m phase). It can be seen that there are weak peaks at 37.5°, 43.6° and 63.3° in the patterns of samples attributed to the secondary phases LixNi1−xO, which is a common phenomenon in the preparation of LiNi0.5Mn1.5O4. As previous literature reports,38–40 when the annealing temperature is above 650 °C, LiNi0.5Mn1.5O4 phase suffers a decomposition reaction as shown in eqn (1), while the LixNi1−xO phase has no negative effects on the electrochemical performance of LiNi0.5Mn1.5O4.41 As a result, oxygen deficiency occurs and to maintain charge balance, some Mn3+ ions appear instead of Mn4+ ions, which is helpful to improve the electronic conductivity of LiNi0.5Mn1.5O4.38 The peaks of LBO cannot be detected, which is probably due to the low proportion of the LBO-glass or its amorphous nature.33| LiNi0.5Mn1.5O4 → αLixNi1−xO + βLiNi0.5−yMn1.5+yO4 + γO2 | (1) |
Fig. 2 shows the SEM images of the pristine and LBO-glass coated LiNi0.5Mn1.5O4. It can be seen that the pristine LiNi0.5Mn1.5O4 possesses a distribution of secondary particles sizing between 2–8 μm, while the LBO-glass coated materials possess a part of smaller secondary particles and slighter particle agglomerations. Thus, the LBO-glass coating prevents the aggregation of nanoparticles, which benefits the improvement of the electrochemical properties.
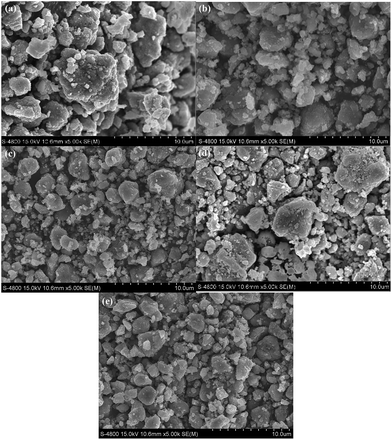 | ||
| Fig. 2 SEM images of (a) LNMO, (b) 0.005LBO–LNMO, (c) 0.01LBO–LNMO, (d) 0.02LBO–LNMO, (e) 0.03LBO–LNMO. | ||
The HRTEM image of the 0.01LBO–LNMO is displayed in Fig. 3. It is observed that a thin film with a thickness in the range of 5–10 nm is coated on the surface of LiNi0.5Mn1.5O4 cathode material. In addition, the fast Fourier transform (FFT) images are shown in the insets of Fig. 3, which confirm the crystalline characteristics of the LiNi0.5Mn1.5O4 and the amorphous structure of the coated LBO-glass layer, in accordance with the XRD analysis. The lattice fringe with a d-spacing value of 0.4765 nm corresponds to the (111) plane of LiNi0.5Mn1.5O4. From the TEM image and the XRD patterns, it is concluded that the LBO-glass layer is amorphous rather than crystalline.33,36
3.2. Electrochemical characterization
Fig. 4 presents the cycling performances at 1 C rate (1 C = 147 mA h g−1) and rate performances of the pristine and LBO-glass coated LiNi0.5Mn1.5O4. As seen in Fig. 4a, the discharge capacities of the samples show an increasing trend along with the cycle number at the initial several cycles. This should be attributed to the activation of the cells, which is a common phenomenon in the study of lithium-ion batteries and can be seen in previously reported literature.42,43 The mass transfer is slower than the charge transfer under high current density. Therefore, during the initial cycles, some active materials are not involved in the charge–discharge process, leading to the low discharge capacities. After a few cycles of activation, the capacities gradually increase due to more and more active materials participating in the charge–discharge process. After several cycles, the LNMO, 0.005LBO–LNMO, 0.01LBO–LNMO, 0.02LBO–LNMO and 0.03LBO–LNMO show the highest discharge capacities of 111.1, 111.5, 111.0, 109.1 and 107.3 mA h g−1, respectively. While after 100 cycles, the corresponding capacities decrease to 94.3, 98.3, 101.5, 98.4 and 95.4 mA h g−1, accompanied with a capacity retention of 85.9%, 88.1%, 91.4%, 90.2% and 89.0% compared with the highest discharge capacity, respectively. For the initial several cycles, the LBO-glass coated materials show lower discharge capacities compared with bare LNMO, which is caused by the electrochemical inactivity of the LBO-glass. However, the LBO-glass coated materials show higher discharge capacities and capacity retentions after 100 cycles at 1 C, indicating that LBO-glass layer plays a protecting role on the surface of the LiNi0.5Mn1.5O4 materials, prevents the side reactions between cathode materials and electrolyte, stabilizes the structure of the cathode materials and thus improves the cycling stability.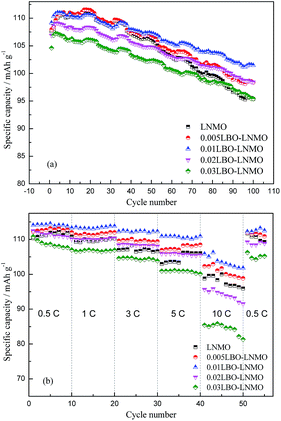 | ||
| Fig. 4 Cycling performances for 100 cycles at 1 C (a) and rate performances (b) of the pristine and LBO-glass coated LiNi0.5Mn1.5O4. | ||
To characterize the rate capability of the samples, electrodes made from the as-prepared materials are charged to 5.0 V at 0.5 C and discharged to 3.5 V at different rates from 0.5 C to 10 C and then back to 0.5 C. It can be clearly seen that the 0.01LBO–LNMO possesses the best rate capability and cyclability. A discharge capacity of 105.8 mA h g−1 can be retained at a discharge rate of 10 C for 0.01LBO–LNMO, while the LNMO, 0.005LBO–LNMO, 0.02LBO–LNMO and 0.03LBO–LNMO deliver lower capacities of 98.7, 102.3, 95.6 and 85.4 mA h g−1, respectively. When the current density is reduced to 0.5 C, the discharge capacities could recover to their initial value, which indicates that rapid lithiation/delithiation and a large current density did not result in structural damage of the crystals. Moreover, both the stable structure and the LBO-glass with fast lithium ion conduction can reduce the polarization especially at high current density, which leads to an improvement of the rate capability. The worse discharge capacity of the 0.02LBO–LNMO and 0.03LBO–LNMO may be induced by the electrochemical inactivity of the excessive LBO-glass. It can be concluded that 1 wt% is a proper coating amount for the LNMO cathode material.
Typical charge–discharge profiles at 0.5 C for the 10th cycle and cyclic voltammogram (CV) curves for the pristine and LBO-coated LiNi0.5Mn1.5O4 at a scanning rate of 0.1 mV s−1 are shown in Fig. 5. As observed in Fig. 5a, two dominant plateaus around 4.7 V belonging to the charge curves are attributed to the redox reactions of Ni3+/Ni2+ and Ni4+/Ni3+.29 While for the discharge curves, only one distinct plateau is found around 4.7 V. As shown in Fig. 5b, two pairs of redox peaks (around 4.76/4.66 V and 4.81/4.67 V) are ascribed to the redox reactions of Ni3+/Ni2+ and Ni4+/Ni3+, which is in accordance with the above results. Besides, a weak peak at around 4.0 V indicates the Mn4+/Mn3+ redox reaction, which is in agreement with the magnified region in the CV curves. What's more, the 0.01LBO–LNMO presents a larger current density than other samples, suggesting a lower polarization of the material. On the basis of previous reports,31,32 the LBO-glass is not a good electronic conductor, but a very good Li+ ionic conductor. The thin coating layer may promote the Li+ transfer process through the interfaces between the LBO-glass and the LNMO materials. As a result, the electrochemical polarization is decreased.
 | ||
| Fig. 5 Typical charge–discharge profiles (a) and cyclic voltammogram (CV) curves at a scanning rate of 0.1 mV s−1 (b) for the pristine and LBO-glass coated LiNi0.5Mn1.5O4. | ||
To further characterize the improved electrochemical behaviour of the 0.01LBO–LNMO, the EIS test results of the LNMO and 0.01LBO–LNMO are shown in Fig. 6. The resistances are evaluated using the equivalent circuit model shown in the inset of the Fig. 6a,23,44,45 and the fitted values are listed in Table 1. According to the literatures,23,45 Re represents the solution resistance; RSEI and CPE1 signify the diffusion resistance of Li+ through the solid-electrolyte interface (SEI) layer and the corresponding constant phase element (CPE); Rct and CPE2 correspond to the charge transfer resistance and the corresponding CPE, while RW (not calculated here) is related to the solid-state diffusion of Li+ in the active materials corresponding to the slope of the line at low frequency. From the fitted data, it can be concluded that the resistances of the electrolyte (Re) are similar for both the LNMO and the 0.01LBO–LNMO. However, after 1 cycle at 1 C, both the RSEI and Rct of the 0.01LBO–LNMO are much lower than those of the LNMO. Further, after the cells are charged and discharged for 30 cycles, RSEI and Rct show an increasement, which of the 0.01LBO–LNMO are still much smaller than that of that LNMO, indicating that the LBO-glass coating layer plays an important role in reducing the resistances. What's more, the EIS can also be used to calculate the lithium-ion diffusion coefficient (D) through employing the following equations:19,26
| Zre = Re + Rct + σω−1/2 | (2) |
 | (3) |
 | ||
| Fig. 6 Nyquist plots (a) and graph of Zre plotted against ω−1/2 (b) of the LNMO and 0.01LBO–LNMO after cycled at 1 C for 1 cycle and 30 cycles. | ||
| Samples | Re/Ω | RSEI/Ω | Rct/Ω | σ/Ω cm2 s−1/2 | D/cm2 s−1 |
|---|---|---|---|---|---|
| LNMO@1st | 4.61 | 269.2 | 223.4 | 100.4 | 9.63 × 10−13 |
| LNMO@30th | 4.01 | 295.3 | 333.1 | 190.7 | 5.07 × 10−13 |
| 0.01LBO–LNMO@1st | 5.48 | 60.4 | 81.1 | 65.6 | 1.47 × 10−12 |
| 0.01LBO–LNMO@30th | 4.43 | 183.5 | 170.3 | 75.6 | 1.28 × 10−12 |
Self-discharge profiles of LNMO and 0.01LBO–LNMO cells are illustrated in Fig. 7. After storage for 10 days, the voltage of the charged LNMO cell decreases from 4.96 V to 4.70 V, while that of the 0.01LBO–LNMO decreases from 4.96 V to 4.74 V. It is apparent that the charged 0.01LBO–LNMO suffers less self-discharge than LNMO. Thus, LBO coating is an effective method to suppress this self-discharge, which may be attributed to the fact that LBO layer is able to protect the active material form reacting with the electrolyte and prevent the self-discharge.
 | ||
| Fig. 7 Self-discharge profiles of LNMO and 0.01LBO–LNMO cells after 5 cycle at 0.1 C in the potential range of 3.5–5.0 V. | ||
DSC spectra of over charged LNMO and 0.01LBO–LNMO are illustrated in Fig. 8. As shown in the Fig. 8, peaks around 100 °C may be attributed to the decomposition of the trace of electrolyte. When the temperature reaches around 200 °C, the cathodes undergo an exothermic reaction with large enthalpy and the reaction of LNMO gets completed at a much lower temperature than that of 0.01LBO–LNMO. Pristine LiNi0.5Mn1.5O4 cathode is found to be thermally unsafe, while the coated one gain more thermal stability, which may be attributed to the fact that LBO glass improves the safety of the cathode material.
 | ||
Fig. 8 DSC spectra of over charged LNMO and 0.01LBO–LNMO cathodes with traces of 1.0 mol L−1 LiPF6 in EC–EMC (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) electrolyte with a heating rate of 10 °C min−1. 7) electrolyte with a heating rate of 10 °C min−1. | ||
4. Conclusions
In summary, the LiNi0.5Mn1.5O4 cathode materials were synthesized by a co-precipitation method and the LBO-glass coated LiNi0.5Mn1.5O4 were synthesized by a solution method followed by heat treatment. The XRD patterns and TEM image indicate that the LBO-glass layer is amorphous. Cycling tests show the 0.01LBO–LNMO possesses the optimal electrochemical stability, which exhibits a capacity retention of 91.4% after 100 cycles at 1 C, higher than that of the LNMO, 0.005LBO–LNMO, 0.02LBO–LNMO and 0.03LBO–LNMO, corresponding 85.9% 88.1%, 90.2% and 89.0%, respectively. Furthermore, rate behaviour tests indicate that the 0.01LBO–LNMO holds the best rate performance and it keeps a discharge capacity of 105.8 mA h g−1 even at 10 C. The EIS and self-discharge results manifest that the LBO-glass layer not only acts as a Li+ conductor, but also protects the electrode from corrosion by the electrolyte and prevents the reactions between electrolyte and cathode materials, reducing the impedance and the self-discharge of the cells. DSC data indicates that LBO coating improves the thermal safety of LiNi0.5Mn1.5O4. Thus, LBO-glass surface modification is a promising way to improve the electrochemical properties of LiNi0.5Mn1.5O4. As a promising extension, this LBO-glass coating method can be employed to improve other lithium-ion batteries materials.Acknowledgements
This work is supported by the project of Innovative group for high-performance lithium-ion power batteries R&D and industrialization of Guangdong Province (Grant No. 2013N079).Notes and references
- J. Chong, S. Xun and J. Zhang, Chem.–Eur. J., 2014, 7479 CrossRef CAS PubMed
.
- J. Molenda, J. Marzec and K. Świerczek, Solid State Ionics, 2004, 171, 215 CrossRef CAS PubMed
.
- T. Yang, N. Zhang and Y. Lang, Electrochim. Acta, 2011, 56, 4058 CrossRef CAS PubMed
.
- L. Yang, B. Ravdel and B. L. Lucht, Electrochem. Solid-State Lett., 2010, 13, A95 CrossRef CAS PubMed
.
- K. Xu, Chem. Rev., 2004, 104, 4303 CrossRef CAS
.
- J. Liu and A. Manthiram, J. Electrochem. Soc., 2009, 156, A833 CrossRef CAS PubMed
.
- J. Arrebola, A. Caballero and L. Hernan, J. Electrochem. Soc., 2007, 154, A178 CrossRef CAS PubMed
.
- Z. Yang, Y. Jiang and J. H. Kim, Electrochim. Acta, 2014, 117, 76 CrossRef CAS PubMed
.
- M. H. Liu, H. T. Huang and C. M. Lin, Electrochim. Acta, 2014, 120, 133 CrossRef CAS PubMed
.
- G. Liu, L. Zhang and L. Sun, Mater. Res. Bull., 2013, 48, 4960 CrossRef CAS PubMed
.
- O. Sha, Z. Tang and S. Wang, Electrochim. Acta, 2012, 77, 250 CrossRef CAS PubMed
.
- G. B. Zhong, Y. Y. Wang and Z. C. Zhang, Electrochim. Acta, 2011, 56, 6554 CrossRef CAS PubMed
.
- J. Liu and A. Manthiram, J. Phys. Chem. C, 2009, 113, 15073 CAS
.
- H. Xu, X. J. Liu and Q. W. Peng, Mater. Express, 2014, 4, 72 CrossRef CAS PubMed
.
- D. Liu, Y. Lu and J. B. Goodenough, J. Electrochem. Soc., 2010, 157, A1269 CrossRef CAS PubMed
.
- M. Mo, K. S. Hui, X. Hong and J. Guo, Appl. Surf. Sci., 2014, 290, 412 CrossRef CAS PubMed
.
- H. Wang, T. A. Tan and P. Yang, J. Phys. Chem. C, 2011, 115, 6102 CAS
.
- M. l. P. Le, P. Strobel and F. Alloin, Electrochim. Acta, 2010, 56, 592 CrossRef CAS PubMed
.
- T. F. Yi, Y. Xie and Y. R. Zhu, J. Power Sources, 2012, 211, 59 CrossRef CAS PubMed
.
- S. J. R. Prabakar, S. C. Han and S. P. Singh, J. Power Sources, 2012, 209, 57 CrossRef CAS PubMed
.
- J. Arrebola, A. Caballero and L. Hernán, J. Power Sources, 2010, 195, 4278 CrossRef CAS PubMed
.
- J. Liu and A. Manthiram, Chem. Mater., 2009, 21, 1695 CrossRef CAS
.
- H. M. Wu, I. Belharouak and A. Abouimrane, J. Power Sources, 2010, 195, 2909 CrossRef CAS PubMed
.
- Y. Lee, J. Mun and D.-W. Kim, Electrochim. Acta, 2014, 115, 326 CrossRef CAS PubMed
.
- Y. Fan, J. Wang and Z. Tang, Electrochim. Acta, 2007, 52, 3870 CrossRef CAS PubMed
.
- X. Li, W. Guo and Y. Liu, Electrochim. Acta, 2014, 116, 278 CrossRef CAS PubMed
.
- Y. Lin, Y. Yang and R. Yu, J. Power Sources, 2014, 259, 188 CrossRef CAS PubMed
.
- H. B. Kang, S. T. Myung and K. Amine, J. Power Sources, 2010, 195, 2023 CrossRef CAS PubMed
.
- D. Liu, Y. Bai and S. Zhao, J. Power Sources, 2012, 219, 333 CrossRef CAS PubMed
.
- G. Amatucci, A. Blyr and C. Sigala, Solid State
Ionics, 1997, 104, 13 CrossRef CAS
.
- M. Eddrief, P. Dzwonkowski and C. Julien, Solid State Ionics, 1991, 45, 77 CrossRef CAS
.
- W. Soppe, F. Aldenkamp and H. Den Hartog, J. Non-Cryst. Solids, 1987, 91, 351 CrossRef CAS
.
- J. Ying, C. Wan and C. Jiang, J. Power Sources, 2001, 102, 162 CrossRef CAS
.
- J. Dou, X. Kang and T. Wumaier, J. Solid State Electrochem., 2012, 16, 1481 CrossRef CAS PubMed
.
- H. Şahan, H. Göktepe and Ş. Patat, Solid State Ionics, 2008, 178, 1837 CrossRef PubMed
.
- H.-W. Chan, J.-G. Duh and S.-R. Sheen, Surf. Coat. Technol., 2004, 188–189, 116 CrossRef CAS PubMed
.
- H. W. Chan, J. G. Duh and S. R. Sheen, Electrochim. Acta, 2006, 51, 3645 CrossRef CAS PubMed
.
- Y. C. Jin, C. Y. Lin and J. G. Duh, Electrochim. Acta, 2012, 69, 45 CrossRef CAS PubMed
.
- L. Wang, H. Li and X. Huang, et al., Solid State Ionics, 2011, 193, 32 CrossRef CAS PubMed
.
- Q. Zhong, A. Bonakdarpour and M. Zhang, et al., J. Electrochem. Soc., 1997, 144, 205 CrossRef CAS PubMed
.
- G. Q. Liu, L. Wen and X. Wang, J. Alloys Compd., 2011, 509, 9377 CrossRef CAS PubMed
.
- G. L. Xu, Y. F. Xu and J. C. Fang, ACS Appl. Mater. Interfaces, 2013, 5, 6316 CAS
.
- F. Fu, G.-L. Xu and Q. Wang, J. Mater. Chem. A, 2013, 1, 3860 CAS
.
- M. Yang, C. Du and Z. Tang, Ionics, 2014, 20, 1039 CrossRef CAS PubMed
.
- J. Xie, X. Zhao and G. Cao, J. Power Sources, 2005, 140, 350 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2015 |


