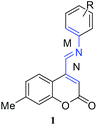
Regiospecific inverse electron demand Diels–Alder reactions of 7-methylcoumarin-4-azadienes†
Kailas K. Sanapa and
Shriniwas D. Samant*b
aDepartment of Chemistry, N.B. Mehta Science College, Bordi, Taluka-Dahanu, Dist-Palghar 400 701, Maharashtra, India
bDepartment of Chemistry, Institute of Chemical Technology, Nathalal Parikh Marg, Matunga, Mumbai 400 019, Maharashtra, India. E-mail: samantsd@yahoo.com; Fax: +91-2233611020; Tel: +91-2233612606
First published on 14th April 2015
Abstract
Condensation of 7-methylcoumarin-4-carbaldehyde with different anilines affords 7-methylcoumarin-4-azadienes. The 7-methylcoumarin-4-azadienes do not undergo normal electron demand Diels–Alder reaction with N-phenylmaleimide, but react with dihydropyran, dihydrofuran, and styrene via inverse electron demand Diels–Alder reaction in the presence of anhydrous ZnCl2. The diene involves the azomethine group and the aniline ring. The product is a mixture of two diastereomers in which the major diastereomer has all the hydrogens at the ring junction in cis configuration.
Introduction
The coumarins are versatile molecules because many of them, natural as well as synthetic, exhibit a broad spectrum of biological properties such as antitumour,1 antimicrobial2 and antiviral3 activities. Polycyclic benzopyrones embedding a coumarin ring also show diverse activities. One of the strategies of building of such molecules is annulation over a coumarin ring. This strategy works very well with the 3,4-double bond in coumarin. The 3,4-double bond functions as a dienophile4 due to activation by adjacent carbonyl groups and it also undergoes an addition reaction.5 3-Vinyl and 4-styryl substituted coumarins perform as dienes and the corresponding Diels–Alder reaction gives 3,4-annulated coumarins.6 Inverse electron demand Diels–Alder reaction of coumarins containing electron deficient diene with electron rich dienophiles is known to give 3,4-annulated coumarins.7The aza Diels–Alder reaction of coumarin-3-azadienes derived from 3-aminocoumarin and its intramolecular version are known.8 Conjugated polyenes containing more than one diene component are interesting substrates for Diels–Alder reaction, as the reaction involves a challenge of regioselection. Diels–Alder reaction of dendralene and biscoumarinylethene types of polyene systems are reported in literature to give regioselective product.9 Diels–Alder reaction of heteropolyene (cross conjugated) system like [3]-3-heterodendralenes (thia-10, oxa-11, and aza-12) with an suitable dienophiles gives diversified heterocycles. More recently Saito et al. reported the Diels–Alder reaction of [3]-1-azadendralenes in which cross-conjugated 1-azatriene underwent an initial hetero Diels–Alder reaction on the 1-aza-1,3-butadiene system with tosyl isocyanate to afford the mono-cycloadduct pyrimidinone which on further subsequent Diels–Alder reaction with dienophiles provides hexahydroquinazolin-2(1H)-ones with high stereoselectivity.13
As a part of our interest in the Diels–Alder reaction of coumarins containing diene component, we thought that coumarin-4-azadienes (1) which contain a 3,4-double bond of coumarin ring conjugated to phenylimino group, would be an interesting diene system to study the Diels–Alder reaction; targeting coumarin containing polycyclic compounds.
Azadiene 1 is an interesting substrate for Diels–Alder reaction as it contains two potentially reactive azadiene: a 2-azadiene (involving the aniline ring i.e. M) and a 1-azadiene (involving the C3–C4 coumarin double bond i.e. N) with different orbital characteristics and electron demands; possibly providing regioselectivity in the reaction. In general, simple 1- and 2-azadienes, due to their electron-deficient nature favor participation in `inverse electron demand' (LUMO diene-controlled) Diels–Alder reaction.14 A major difference between the two systems is the efficacy of Lewis acid catalysis in the 2-azadiene cycloadditions. However reactivity of both the azadienes can be tuned by introducing either electron-withdrawing or electron-donating substituents at proper positions of the diene.15 The introduction of electron-withdrawing substituents at the 2, 3, or 4 positions of the 1-azadiene may further accelerate reaction rates through LUMO diene-controlled pathway. Sufficiently electron-donating substituents placed at the C-1 and/or C-3 position in the 2-azadiene can enhance the Diels–Alder reactivity through the HOMO diene-controlled pathway.15 Herein, we report a comprehensive study on the inverse electron demand Diels–Alder reaction of 7-methylcoumarin-4-azadienes (1).
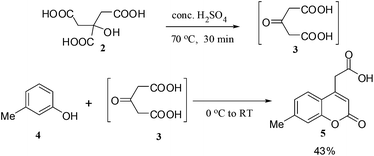
7-Methylcoumarin-4-acetic acid (5) was prepared by the condensation of m-cresol (4) with acetone dicarboxylic acid (3), which in turn was prepared in situ by reacting citric acid (2) with conc. H2SO4 (Scheme 1).16
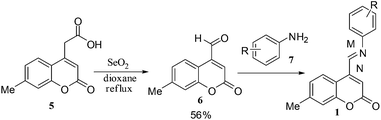
The compound 5 was subjected to oxidation using SeO2 to obtain 7-methylcoumarin-4-carbaldehyde (6), which on condensation with anilines (7) gave 7-methylcoumarin-4-azadienes (1) (Scheme 2).17
Diels–Alder reaction of 4-styrylcoumarins with N-phenylmaleimide (NPMA) (8) is known to give 2,11-diphenyl-3a,10,11,11a-tetrahydro[1]benzopyrano[3,4-e]isoindole-1,3,4(2H)-triones.18 Taking inspiration from this, we carried out the Diels–Alder reaction of 1a (R = H) with NPMA (8) at different temperatures in dioxane, nitrobenzene, and o-dichlorobenzene (o-DCB). In dioxane 1a remained unconsumed, while in nitrobenzene and o-DCB it decomposed to form other side products (TLC). The same reaction under microwave irradiation at different temperatures also failed to give the desired product. The use of Lewis acid (ZnCl2, AlCl3, BF3·OEt2) catalysts (thermal and MW conditions) did not show any further beneficial effect on the reaction. Thus, 1a did not undergo normal electron demand Diels–Alder reaction.
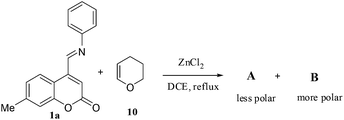
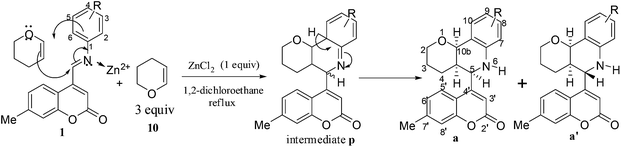
Azadienes are known to undergo Diels–Alder reaction by inverse electron demand pathway. Hence, the reaction of 1a with electron rich dienophiles was carried out. Accordingly, when 3,4-dihydro-2H-pyran (DHP) (1 equiv.) (10) was reacted with 1a, in the presence of ZnCl2 (1 equiv.), a mixture of compounds A (less polar) and B (more polar) was obtained (Scheme 3).
A and B were separated and purified by flash column chromatography on silica gel using chloroform. Gratifyingly, a mixture of A and B was isolated in 67% yield with A (169 mg, 49%) and B (62 mg, 18%). Six different products are possible for this reaction as shown in Fig. 1.
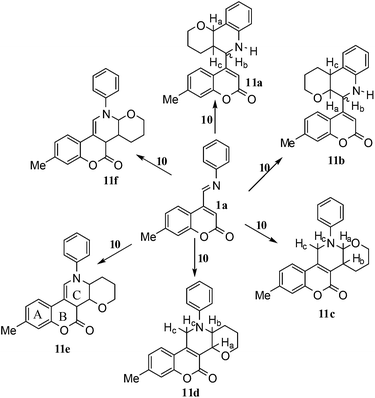 | ||
| Fig. 1 Possible products in the aza Diels–Alder reaction of 7-methylcoumarin-4-azadiene (1a) with dihydropyran (10). | ||
The IR spectra of compounds A and B showed secondary NH group, as sharp medium intensity peaks at 3348 and 3322 cm−1 respectively, and the carbonyl groups showed peaks at 1720 and 1708 cm−1 respectively. 1H NMR spectra of A and B had ratio of aliphatic protons to aromatic protons 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (without considering 3 protons of methyl group). Based on these observations, structures 11e and 11f were ruled out, as they contain the ratio 9
8 (without considering 3 protons of methyl group). Based on these observations, structures 11e and 11f were ruled out, as they contain the ratio 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
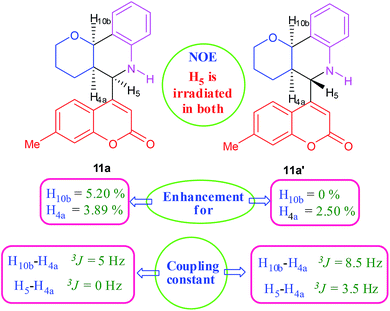
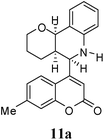
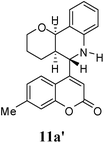
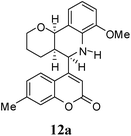
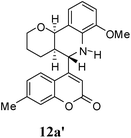
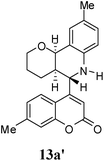
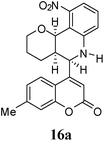
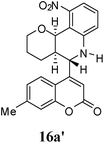
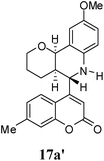
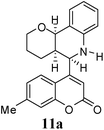
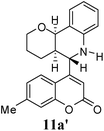
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9. Moreover, in 11e and 11f the newly formed double bond remain in ring C, exocyclic to ring B, which we have shown earlier to be improbable.18 In 11c and 11d, NH is absent and Hc is expected to give two closely spaced doublets around 3.0–3.5 δ. This feature was not seen in the 1H NMR spectra of A and B. Hence, 11c and 11d were ruled out. Among 11a and 11b, the distinguishing proton appears to be Ha, which in the case of 11b would come downfield compared to that in 11a. In the D2O exchanged 1H NMR spectra of A and B, the NH peak disappeared and DHO peak appeared at 4.7 δ. Hence, the structures 11c and 11d were ruled out, as they do not contain NH proton. Based on 13C NMR spectra structure 11c was ruled out, as it contains a carbon atom which is flanked by two electronegative atoms, oxygen and nitrogen, and is expected to give a peak around 80–85 δ. Such a peak was not observed in both the spectra. To throw more light on the structure, DQF-COSY spectra of A and B were recorded and the spectra supported structure 11a. Based on the stereochemical restriction in the Diels–Alder reaction, compound 11a can exists in two diastereomeric forms 11a and 11a′. The most diagnostic parameter for the structural assignment is the scalar coupling constant between protons H4a and H5. As depicted in Fig. 2, the cis isomer has small coupling constants JH-5,H-4a = 0 Hz, JH-10b,H-4a = 5 Hz, consistent with an all cis configuration of the hydrogen atoms of 4a, 5, and 10b positions. In the trans isomer, the value of JH-5,H-4a = 8.5 Hz is large and indicates the anti orientation of the hydrogen atoms of 4a and 5 positions. To get more clarification about the stereochemical relationship NOE spectra were recorded. In both the spectra the signal corresponding to H5 (5.10 δ in compound A, 4.56 δ in compound B) was irradiated. In the case of A the irradiation resulted in intensification of H4a by 3.89% and H10b by 5.20%; which confirmed the cis arrangement between H5, H4a and H10b. In the case of B there was enhancement of H4a by 2.50% and there was no NOE observed for H10b, because of trans relationship between H5–H4a and H5–H10b (Fig. 2). By observing the NOE effect it was clear that in the less polar compound (A = 11a) the stereochemical relation between H5 and H4a was cis, while in the more polar compound (B = 11a′) the relation was trans.
9. Moreover, in 11e and 11f the newly formed double bond remain in ring C, exocyclic to ring B, which we have shown earlier to be improbable.18 In 11c and 11d, NH is absent and Hc is expected to give two closely spaced doublets around 3.0–3.5 δ. This feature was not seen in the 1H NMR spectra of A and B. Hence, 11c and 11d were ruled out. Among 11a and 11b, the distinguishing proton appears to be Ha, which in the case of 11b would come downfield compared to that in 11a. In the D2O exchanged 1H NMR spectra of A and B, the NH peak disappeared and DHO peak appeared at 4.7 δ. Hence, the structures 11c and 11d were ruled out, as they do not contain NH proton. Based on 13C NMR spectra structure 11c was ruled out, as it contains a carbon atom which is flanked by two electronegative atoms, oxygen and nitrogen, and is expected to give a peak around 80–85 δ. Such a peak was not observed in both the spectra. To throw more light on the structure, DQF-COSY spectra of A and B were recorded and the spectra supported structure 11a. Based on the stereochemical restriction in the Diels–Alder reaction, compound 11a can exists in two diastereomeric forms 11a and 11a′. The most diagnostic parameter for the structural assignment is the scalar coupling constant between protons H4a and H5. As depicted in Fig. 2, the cis isomer has small coupling constants JH-5,H-4a = 0 Hz, JH-10b,H-4a = 5 Hz, consistent with an all cis configuration of the hydrogen atoms of 4a, 5, and 10b positions. In the trans isomer, the value of JH-5,H-4a = 8.5 Hz is large and indicates the anti orientation of the hydrogen atoms of 4a and 5 positions. To get more clarification about the stereochemical relationship NOE spectra were recorded. In both the spectra the signal corresponding to H5 (5.10 δ in compound A, 4.56 δ in compound B) was irradiated. In the case of A the irradiation resulted in intensification of H4a by 3.89% and H10b by 5.20%; which confirmed the cis arrangement between H5, H4a and H10b. In the case of B there was enhancement of H4a by 2.50% and there was no NOE observed for H10b, because of trans relationship between H5–H4a and H5–H10b (Fig. 2). By observing the NOE effect it was clear that in the less polar compound (A = 11a) the stereochemical relation between H5 and H4a was cis, while in the more polar compound (B = 11a′) the relation was trans.
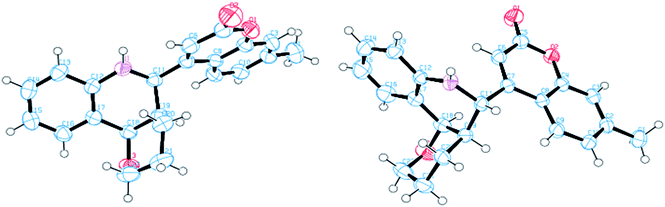
To establish the structures unequivocally, single crystal XRD was recorded on a Bruker axs kappa apex 2 CCD Diffractometer using graphite monochromated Mo Kα radiation (λ = 0.71073 Å). The ORTEP diagram of A and B are shown in Fig. 3. Thus the two compounds, A and B, are diastereomers of each other.
Compound A (11a) is 4aR*, 5R*, 10bR*-5-(7-methylcoumarin-4-yl)-3,4,4a,5,6,10b-hexahydro-2H-pyrano[3,2-c]quinoline. Compound B (11a′) is 4aR*, 5S*, 10bR*-5-(7-methylcoumarin-4-yl)-3,4,4a,5,6,10b-hexahydro-2H-pyrano[3,2-c]quinoline.
In structure 1, diene N appears to be more electron deficient than diene M. However, in the case of an unsymmetrical polyene system, particularly the one like in the present case, where there is a combination of heteroatoms, electron donating groups and electron withdrawing groups, the regioselection is difficult to predict. To through more light on the selectivity, 6 was condensed with benzylamine to obtain corresponding anil (1i). 1i is devoid of diene component M and has only diene component N. 1i failed to react with NPMA as well as DHP.
A series of 7-methylcoumarin-4-azadienes were prepared as per literature method by reacting 7-methylcoumarin-4-carbaldehyde (6) and anilines (7) in benzene at reflux condition (Table 1).17 All anilines (7) react with 7-methylcoumarin-4-carbaldehyde (6) to give high yield of 1. Primary aliphatic benzyl amine reacts very rapidly as compared to other aromatic amines (entry 9). Due to electron deficient nature of 3-nitroaniline (1f) and 4-nitroaniline (1e) it took maximum time for condensation (entry 5 & 6). As expected steric effect is pronounced in the reaction of 1h with 6, reaction required more time for completion (entry 8).
| Entry | R | Product (1) | Timeb (min) | Yieldc of 1 (%) |
|---|---|---|---|---|
| a Reaction conditions: 7-methylcoumarin-4-carbaldehyde (1 mmol), aniline (1 mmol), benzene (5 mL), reflux temp.b Time required for total consumption of 6.c Isolated yield. | ||||
| 1 | H | 1a | 60 | 83 |
| 2 | 2-OMe | 1b | 30 | 84 |
| 3 | 4-Me | 1c | 30 | 86 |
| 4 | 4-Br | 1d | 40 | 85 |
| 5 | 4-NO2 | 1e | 120 | 72 |
| 6 | 3-NO2 | 1f | 110 | 76 |
| 7 | 4-OMe | 1g | 30 | 89 |
| 8 | 2,6-(Me)2 | 1h | 110 | 80 |
| 9 | — | 1i | 20 | 84 |
The effect of Lewis acid catalyst and solvent on the reaction was then investigated (Table 2). The reaction of 1a and DHP (10) could not be effected without a catalyst. Different catalysts were used for the reaction in 1,2-dichloroethane. Anhydrous Lewis acids were very effective in catalyzing the reaction, while hydrated Lewis acids were much less active. Bronsted acids also were not very effective in catalyzing the reaction. In the case of AlCl3 and FeCl3, the reaction at 0 °C gave about 50–55% combined yield of 11a and 11a′. When the same reaction was carried out at room temperature, the combined yield of 11a and 11a′ decreased and substantial amount of a sticky material was formed, which was insoluble in organic solvents. SnCl4 was also effective. ZnCl2 was found to be not only effective, but was very convenient to use. At room temperature the reaction was slow and the combined yield was 77% (entry 8). At reflux condition the reaction was fast and gave 83% of yield (entry 9). Hence, all the reactions were carried out at the reflux temperature. CAN was also effective and in 3 h, 54% of combined yield was obtained. Except CAN, with all the catalysts 11a was obtained as the major product, while with CAN, 11a′ was the major product.
| Entry | Catalystb | Condition (time)c | Yieldd (%) | |
|---|---|---|---|---|
| 11a | 11a′ | |||
| a Reaction conditions: 7-methylcoumarin-4-azadiene (1 mmol), dihydropyran (3 mmol), 1,2-dichloroethane (5 mL).b Catalyst (1 mmol).c Time for which reaction was continued.d Isolated yield. | ||||
| 1 | Without catalyst | Reflux (24 h) | NR | |
| 2 | BF3·OEt2 | Rt (15 min) | 57 | 10 |
| 3 | BF3·OEt2 | Reflux (5 min) | 44 | 8 |
| 4 | AlCl3 | 0 °C (2 h) | 40 | 10 |
| 5 | FeCl3 | 0 °C (2 h) | 32 | 22 |
| 6 | AlCl3 | Rt (5 min) | 31 | 06 |
| 7 | FeCl3 | Rt (5 min) | 24 | 16 |
| 8 | ZnCl2 | Rt (9 h) | 59 | 18 |
| 9 | ZnCl2 | Reflux (3 h) | 60 | 23 |
| 10 | AlCl3·6H2O | Rt (25 min) | 27 | 08 |
| 11 | FeCl3·6H2O | Rt (15 min) | 29 | 05 |
| 12 | ZnCl2·2H2O | Reflux (10 h) | 33 | 14 |
| 13 | Methanolic HCl | Rt (14 h) | 12 | 18 |
| 14 | Conc. H2SO4 | 0 °C (5 min) | 20 | — |
| 15 | SnCl4 | Rt (15 min) | 46 | 14 |
| 16 | CAN | Rt (3 h) | 20 | 34 |
Based on this study, ZnCl2 was selected as the catalyst for the reaction and using this catalyst different solvents such as methanol, ethanol, acetonitrile, dioxane, tetrahydrofuran, 1,2-dichloroethane and toluene were tried. Except, 1,2-dichloroethane and acetonitrile the reaction did not take place in other solvents. 1,2-Dichloroethane was the best solvent found for reaction and gave 83% of combined yield of 11a and 11a′.
At this stage, other parameters, i.e. catalyst loading and molar ratio of the reactants were studied (Table 3). The yield of the product considerably improved (83%) using 3 equiv. of DHP. Further increase in the amount of DHP did not increase the yield (entries 8 and 9). Also the yield was not significantly increased by increasing the amount of 7-methylcoumarin-4-azadiene (1a) with respect to dienophile. The yield was considerably improved (67%) when one equivalent of ZnCl2 was used (entry 2). Further increase in the catalyst loading did not improve the yield (entries 3 and 4).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 on the reaction of 1a and 10a
10 on the reaction of 1a and 10a
| Entry | ZnCl2 (equiv.) | Molar ratio (1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) 10) |
Timeb (h) | % Yieldc (11a + 11a′) | Combined yield (11a + 11a′) (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 7-methylcoumarin-4-azadiene (1 mmol), 1,2-dichloroethane (5 mL), reflux temp.b Time for which reaction was continued.c Isolated yield. | |||||
| 1 | 0.5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6 | 39 + 14 | 53 |
| 2 | 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6 | 49 + 18 | 67 |
| 3 | 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6 | 51 + 18 | 69 |
| 4 | 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6 | 42 + 18 | 60 |
| 5 | 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6 | 46 + 20 | 66 |
| 6 | 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
5 | 52 + 23 | 75 |
| 7 | 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
3 | 60 + 23 | 83 |
| 8 | 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
3 | 61 + 20 | 81 |
| 9 | 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
3 | 61 + 21 | 82 |
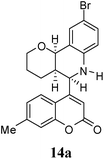
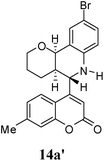
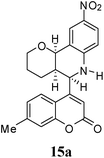
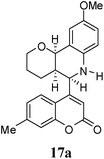
Under the optimized conditions, different 7-methylcoumarin-4-azadienes (1a–1g) were reacted with DHP (10) in the presence of ZnCl2 to obtain the corresponding aza Diels–Alder adducts (Scheme 4, Table 4). All the dienes were reactive and gave the adducts in good yield; however, the time required for complete consumption of the diene varied substantially from diene to diene. The reaction with highly electron deficient diene (1e) was very fast, however we were unable to isolate the more polar product 15a′ and only less polar product 15a was isolated in low yield. Reaction of electron rich dienes 1b and 1g, gave high percentage of a. The reaction of 1h and 1i did not take place even up to 18 h. It also supported that adduct was derived from the M diene system. In all the cases “a” was obtained as the major isomer; the highest being found in the case of dienes 1a and 1b.
| Entry | Azadiene (1) | Products | Timeb (h) | Ratioc (a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a′) (%) a′) (%) |
Yieldd a (%) | Yieldd a′ (%) | |
|---|---|---|---|---|---|---|---|
| (a) | (a′) | ||||||
| a Reaction conditions: 7-methylcoumarin-4-azadiene (1 mmol), dihydropyran (3 mmol), 1,2-dichloroethane (5 mL), Reflux temp, ZnCl2 (1 mmol).b Time for which reaction was continued.c Ratio a/a′ obtained by isolating both the products.d Isolated yield.e Reaction was carried out at room temperature. | |||||||
| 1 | 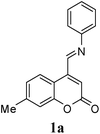 |
 |
 |
3 | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
60 | 23 |
| 2 | 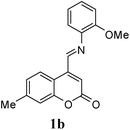 |
 |
 |
4.5 | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
68 | 10 |
| 3 |  |
 |
 |
4 | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 44 |
45 | 35 |
| 4 | 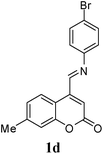 |
 |
 |
4 | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 44 |
43 | 34 |
| 5e | 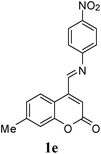 |
 |
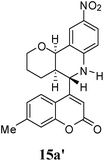 |
10 | — | 36 | Unable to isolate |
| 6 | 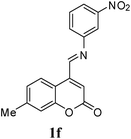 |
 |
 |
1 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
45 | 20 |
| 7 |  |
 |
 |
4 | 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41 41 |
47 | 33 |
| 8 | 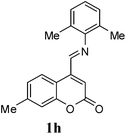 |
No reaction | 18 | ||||
| 9 | 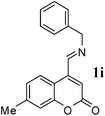 |
No reaction | 18 | ||||
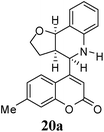
Other dienophiles like dihydrofuran (DHF) (18) and styrene (19) were reacted with 1a under the similar conditions (Table 5). As expected, the reaction proceeded rapidly with electron rich dienophile; the order being: styrene < DHP < DHF. The reaction with DHF was very fast as compared to that with DHP. The diastereomeric ratio was almost the same in the case of DHP and DHF, but changed when the styrene was the dienophile. In the case of DHF, we were unable to isolate the more polar product 20a′ and only less polar 20a was isolated in low yield.
| Entry | Dienophile | Products | Timeb (h) | Ratioc (a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a′) (%) a′) (%) |
Yieldd a (%) | Yieldd a′ (%) | |
|---|---|---|---|---|---|---|---|
| (a) | (a′) | ||||||
| a Reaction conditions: 7-methylcoumarin-4-azadiene (1 mmol), dienophile (3 mmol), 1,2-dichloroethane (5 mL), reflux temp, ZnCl2 (1 mmol).b Time for which reaction was continued.c Ratio a/a′ obtained by isolating both the product.d Isolated yield.e Ratio a/a′ obtained from mixture of 1H NMR. | |||||||
| 1 | 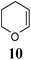 |
 |
 |
3 | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
60 | 23 |
| 2e |  |
 |
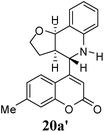 |
1.5 | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 34 |
30 | Unable to isolate |
| 3 |  |
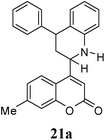 |
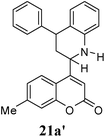 |
4 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
48 | 32 |
To ascertain whether the adducts equilibrated, diastereomer 11a was subjected to the same reaction conditions, i.e. 1 equivalent of ZnCl2 in dichloroethane. No sign of conversion to the diastereomer 11a′ was observed at room temperature (3 h) or even after 24 h at reflux. Also, a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 mixture of the diastereomers (11a + 11a′) did not show any change in the ratio when heated to reflux in 1,2-dichloroethane in the presence of 1 equiv. of ZnCl2. These results suggested that the 1,3-prototropic shift is not reversible under the reaction conditions.
50 mixture of the diastereomers (11a + 11a′) did not show any change in the ratio when heated to reflux in 1,2-dichloroethane in the presence of 1 equiv. of ZnCl2. These results suggested that the 1,3-prototropic shift is not reversible under the reaction conditions.
In the Diels–Alder reaction of 2-azadiene; both the concerted and stepwise mechanism have been postulated.19 It has also been postulated that the reaction mechanism depends on relative face of approach of reactants, nature of the solvent and dienophile used.19b,20 In a stepwise mechanism it is postulated that dipolar intermediate can be trapped by nucleophiles like methanol, acetic acid.21 To ascertain the mechanism of the reaction, an azadiene 1a was reacted with 10 using optimized reaction conditions. Addition of nucleophilic solvent like methanol, acetic acid in the reaction of 1a and 10 does not have any effect on trapping of polar reaction intermediate which support concerted mechanism for the reaction.
Experimental
7-Methylcoumarin-4-acetic acid, 7-methylcoumarin-4-carbaldehyde, 7-methylcoumarin-4-azadienes were prepared by following the reported methods.16,17 1,2-Dichloroethane was freshly distilled from calcium hydride. The products were separated and purified by column chromatography using 100–200 mesh silica gel. Melting points were determined on an Analab melting point apparatus (Model-μThermocal 10) in open capillary tubes and are uncorrected. The IR spectra were recorded on a Jasco-4100 spectrophotometer. 1H NMR spectra were recorded on 300 MHz or 500 MHz spectrometers. 13C NMR spectra were recorded on 75 MHz or 100 MHz. Chemical shifts are reported in parts per million relative to the central line of the multiplet at 77.0 ppm for CDCl3, 39.5 ppm for DMSO. The mass spectra were recorded on a Finnigan LCQ Advantage Max spectrometer. Elemental analysis was carried out with a Thermo finnigan, Flash EA 1112 instrument.General procedure for the synthesis coumarin-4-azadienes (8)
7-Methylcoumarin-4-carbaldehyde (6) (1 mmol), and aniline (7) (1 mmol) were refluxed in benzene (5 mL) for an appropriate time shown in Table 1. After complete consumption of 6, the solution was cooled to room temperature. The solid was separated by filtration, followed by washing with cold benzene and hexane. The mother liquor and washings were combined and concentrated under reduced pressure. The product 8 was purified by recrystallization from ethyl acetate.General procedure for the aza Diels–Alder reaction between 1 and 10
7-Methylcoumarin-4-azadiene (1) (1 mmol) and anhydrous ZnCl2 (0.136 g, 1 mmol) were stirred in 1,2-dichloroethane (5 mL) for 15 min and dihydropyran (10) (0.252 g, 3 mmol) was added slowly at room temperature. The solution was heated to reflux till complete consumption of 1. The solution was cooled to room temperature, quenched with water (10 mL) and extracted with chloroform (10 mL). The extract was dried over anhydrous Na2SO4. The decanted organic layer was evaporated to obtain a sticky mass which was purified by column chromatography on silica gel using chloroform.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1622, 1600 and 1547 (aromatic C
O); 1622, 1600 and 1547 (aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C
C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); 1H NMR (300 MHz, CDCl3): δ 6.78 (s, 1H, C3H), 7.18 (d, 1H, C6H, J = 8.4 Hz), 8.66 (s, 1H, H–C
N); 1H NMR (300 MHz, CDCl3): δ 6.78 (s, 1H, C3H), 7.18 (d, 1H, C6H, J = 8.4 Hz), 8.66 (s, 1H, H–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-), 7.28–7.38 (m, 3H, C4′–H, C5′–H and C6′-H), 7.45–7.50 (t, 2H, C3′–H and C7′–H, J = 7.5 Hz), 8.67 (d, 1H, C5–H, J = 8.4 Hz).
N-), 7.28–7.38 (m, 3H, C4′–H, C5′–H and C6′-H), 7.45–7.50 (t, 2H, C3′–H and C7′–H, J = 7.5 Hz), 8.67 (d, 1H, C5–H, J = 8.4 Hz).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1615, 1588 and 1553 (aromatic C
O); 1615, 1588 and 1553 (aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1478, 1321, 1252, 1139, 1073, 754 cm−1; 1H NMR (500 MHz, CDCl3): δ 1.41–1.61 (m, 4H, C3–H and C4–H), 2.47 (m, 1H, C4a–H), 2.53 (s, 3H, CH3) 3.48 (td, 1H, C2a–H, J = 2.5 and 10.5 Hz), 3.66 (dd, 1H, C2b–H, J = 2.5 and 10.5 Hz), 3.82 (bs, 1H, NH), 5.10 (s, 1H, C5–H), 5.47 (d, 1H, C10b–H, J = 5 Hz), 6.74 (d, 1H, C7–H, J = 8 Hz), 6.77 (s, 1H, C3′–H), 6.93 (t, 1H, C9–H, J = 7.5 Hz), 7.18–7.21 (m, 2H, C8–H and C6′–H), 7.26 (s, 1H, C8′–H), 7.51 (d, 2H, C10–H and C5′–H, J = 8 Hz); 13C NMR (75 MHz, CDCl3): 18.5 (C3), 21.5 (CH3), 25.2 (C4), 35.6 (C4a), 54.3 (C5), 60.4 (C2), 72.1 (C10b), 112.1 (C3′), 114.9 (C4a′), 115.3 (C8′), 117.8 (C7), 119.2 (C9), 119.9 (C10a), 122.9, 125.6, 127.5, 128.3, 143.2 (C7′), 144.3 (C6a), 154.0 (C4′ and C8a′), 160.8 (C2′); MS = 348.5 (M + H); anal. calcd for C22H21NO3 (347.41): C, 76.06; H, 6.09; N, 4.03% found: C, 76.19; H, 6.04; N, 4.11%.
C); 1478, 1321, 1252, 1139, 1073, 754 cm−1; 1H NMR (500 MHz, CDCl3): δ 1.41–1.61 (m, 4H, C3–H and C4–H), 2.47 (m, 1H, C4a–H), 2.53 (s, 3H, CH3) 3.48 (td, 1H, C2a–H, J = 2.5 and 10.5 Hz), 3.66 (dd, 1H, C2b–H, J = 2.5 and 10.5 Hz), 3.82 (bs, 1H, NH), 5.10 (s, 1H, C5–H), 5.47 (d, 1H, C10b–H, J = 5 Hz), 6.74 (d, 1H, C7–H, J = 8 Hz), 6.77 (s, 1H, C3′–H), 6.93 (t, 1H, C9–H, J = 7.5 Hz), 7.18–7.21 (m, 2H, C8–H and C6′–H), 7.26 (s, 1H, C8′–H), 7.51 (d, 2H, C10–H and C5′–H, J = 8 Hz); 13C NMR (75 MHz, CDCl3): 18.5 (C3), 21.5 (CH3), 25.2 (C4), 35.6 (C4a), 54.3 (C5), 60.4 (C2), 72.1 (C10b), 112.1 (C3′), 114.9 (C4a′), 115.3 (C8′), 117.8 (C7), 119.2 (C9), 119.9 (C10a), 122.9, 125.6, 127.5, 128.3, 143.2 (C7′), 144.3 (C6a), 154.0 (C4′ and C8a′), 160.8 (C2′); MS = 348.5 (M + H); anal. calcd for C22H21NO3 (347.41): C, 76.06; H, 6.09; N, 4.03% found: C, 76.19; H, 6.04; N, 4.11%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1619, 1533 and 1494 (aromatic C
O); 1619, 1533 and 1494 (aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1315, 1268, 1144, 1067, 756 cm−1; 1H NMR (500 MHz, CDCl3): δ 1.53–1.56 (m, 1H, C3a–H), 1.68–1.91 (m, 3H, C3b–H and C4–H), 2.47 (bs, 1H, C4a–H), 2.52 (s, 3H, CH3) 3.75 (td, 1H, C2a–H, J = 3.5 and 10.5 Hz), 4.01 (bd, 1H, C2b–H), 4.25 (bs, 1H, NH), 4.56 (d, 1H, C5–H, J = 3.5 Hz), 4.97 (d, 1H, C10b–H, J = 8.5 Hz), 6.54 (s, 1H, C3′–H), 6.66 (d, 1H, C7–H, J = 8 Hz), 6.85 (t, 1H, C9–H, J = 7 Hz), 7.16 (d, 1H, C6′–H, J = 8.0 Hz), 7.20 (dt, 1H, C8–H, J = 1.5 and 7 Hz), 7.26 (s, 1H, C8′–H), 7.35 (dd, 1H, C10–H, J = 1.5 and 7.0 Hz), 7.88 (bs, 1H, C5′–H); 13C NMR (75 MHz, CDCl3): 21.6 (CH3), 23.0 (C3), 24.3 (C4), 36.5 (C4a), 53.0 (C5), 66.1 (C2), 72.3 (C10b), 113.8 (C3′), 114.2 (C4a′), 115.4 (C8′), 117.7 (C7), 118.3 (C9), 119.5 (C8), 124.6 (C10a), 125.4, 129.3, 130.0, 143.2 (C6a), 143.3 (C7′), 154.2 (C4′), 156.2 (C8a′), 161.0 (C2′); MS = 348.5 (M + H); anal. calcd for C22H21NO3 (347.41): C, 76.06; H, 6.09; N, 4.03% found: C, 76.14; H, 6.13; N, 3.96%.
C); 1315, 1268, 1144, 1067, 756 cm−1; 1H NMR (500 MHz, CDCl3): δ 1.53–1.56 (m, 1H, C3a–H), 1.68–1.91 (m, 3H, C3b–H and C4–H), 2.47 (bs, 1H, C4a–H), 2.52 (s, 3H, CH3) 3.75 (td, 1H, C2a–H, J = 3.5 and 10.5 Hz), 4.01 (bd, 1H, C2b–H), 4.25 (bs, 1H, NH), 4.56 (d, 1H, C5–H, J = 3.5 Hz), 4.97 (d, 1H, C10b–H, J = 8.5 Hz), 6.54 (s, 1H, C3′–H), 6.66 (d, 1H, C7–H, J = 8 Hz), 6.85 (t, 1H, C9–H, J = 7 Hz), 7.16 (d, 1H, C6′–H, J = 8.0 Hz), 7.20 (dt, 1H, C8–H, J = 1.5 and 7 Hz), 7.26 (s, 1H, C8′–H), 7.35 (dd, 1H, C10–H, J = 1.5 and 7.0 Hz), 7.88 (bs, 1H, C5′–H); 13C NMR (75 MHz, CDCl3): 21.6 (CH3), 23.0 (C3), 24.3 (C4), 36.5 (C4a), 53.0 (C5), 66.1 (C2), 72.3 (C10b), 113.8 (C3′), 114.2 (C4a′), 115.4 (C8′), 117.7 (C7), 118.3 (C9), 119.5 (C8), 124.6 (C10a), 125.4, 129.3, 130.0, 143.2 (C6a), 143.3 (C7′), 154.2 (C4′), 156.2 (C8a′), 161.0 (C2′); MS = 348.5 (M + H); anal. calcd for C22H21NO3 (347.41): C, 76.06; H, 6.09; N, 4.03% found: C, 76.14; H, 6.13; N, 3.96%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1611, 1553 and 1504 (aromatic C
O); 1611, 1553 and 1504 (aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1330, 1254, 1172, 1138, 1087, 866, 815 cm−1; 1H NMR (500 MHz, CDCl3): δ 1.44 (bd, 1H, C3a–H), 1.55–1.67 (m, 3H, C3b–H and C4–H), 2.49–2.51 (m, 1H, C4a–H), 2.55 (s, 3H, CH3), 3.52 (td, 1H, C2a–H, J = 2.5 and 10.5 Hz), 3.68 (dd, 1H, C2b–H, J = 2.5 and 10.5 Hz), 3.95 (s, 3H, OCH3), 4.26 (bs, 1H, NH), 5.08 (s, 1H, C5–H), 5.51 (d, 1H, C10b–H, J = 5 Hz), 6.83 (d, 1H, C10–H, J = 7.5 Hz), 6.88–6.91 (m, 2H, C3′–H and C9–H), 7.17 (d, 1H, C8–H, J = 7.5 Hz), 7.21 (d, 1H, C6′–H, J = 8 Hz), 7.29 (s, 1H, C8′–H), 7.53 (d, 1H, C5′–H, J = 8 Hz); 13C NMR (75 MHz, CDCl3): δ 18.7 (C3), 21.6 (CH3), 25.2 (C4), 35.6 (C4a), 54.2 (OCH3) 55.5 (C5), 60.7 (C2), 72.3 (C10b), 108.6 (C8), 112.4 (C3′), 115.0 (C4a′), 117.9 (C8′), 118.1 (C7), 118.1 (C9), 119.3 (C10), 120.0 (C10a), 122.9 (C6′), 125.6 (C5′), 134.0 (C6a), 143.3 (C7′), 146.7 (C7), 154.0 (C4′), 154.1 (C8a′), 161.0 (C2′); MS = 378.5 (M + H); anal. calcd for C23H23NO4 (377.43): C, 73.19; H, 6.14; N, 3.71%. Found: C, 73.03; H, 6.19; N, 3.57%.
C); 1330, 1254, 1172, 1138, 1087, 866, 815 cm−1; 1H NMR (500 MHz, CDCl3): δ 1.44 (bd, 1H, C3a–H), 1.55–1.67 (m, 3H, C3b–H and C4–H), 2.49–2.51 (m, 1H, C4a–H), 2.55 (s, 3H, CH3), 3.52 (td, 1H, C2a–H, J = 2.5 and 10.5 Hz), 3.68 (dd, 1H, C2b–H, J = 2.5 and 10.5 Hz), 3.95 (s, 3H, OCH3), 4.26 (bs, 1H, NH), 5.08 (s, 1H, C5–H), 5.51 (d, 1H, C10b–H, J = 5 Hz), 6.83 (d, 1H, C10–H, J = 7.5 Hz), 6.88–6.91 (m, 2H, C3′–H and C9–H), 7.17 (d, 1H, C8–H, J = 7.5 Hz), 7.21 (d, 1H, C6′–H, J = 8 Hz), 7.29 (s, 1H, C8′–H), 7.53 (d, 1H, C5′–H, J = 8 Hz); 13C NMR (75 MHz, CDCl3): δ 18.7 (C3), 21.6 (CH3), 25.2 (C4), 35.6 (C4a), 54.2 (OCH3) 55.5 (C5), 60.7 (C2), 72.3 (C10b), 108.6 (C8), 112.4 (C3′), 115.0 (C4a′), 117.9 (C8′), 118.1 (C7), 118.1 (C9), 119.3 (C10), 120.0 (C10a), 122.9 (C6′), 125.6 (C5′), 134.0 (C6a), 143.3 (C7′), 146.7 (C7), 154.0 (C4′), 154.1 (C8a′), 161.0 (C2′); MS = 378.5 (M + H); anal. calcd for C23H23NO4 (377.43): C, 73.19; H, 6.14; N, 3.71%. Found: C, 73.03; H, 6.19; N, 3.57%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1620, 1556 and 1503 (aromatic C
O); 1620, 1556 and 1503 (aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1299, 1266, 1187, 1134, 1085, 865, 822 cm−1; 1H NMR (500 MHz, CDCl3): δ 1.57–1.60 (m, 1H, C3a–H), 1.72–1.82 (m, 3H, C3b–H and C4–H), 2.49 (bs, 1H, C4a–H), 2.54 (s, 3H, CH3), 3.76 (td, 1H, C2a–H, J = 2.5 and 10.5 Hz), 3.90 (s, 3H, OCH3), 4.00 (bd, 1H, C2b–H), 4.25 (bs, 1H, NH), 4.62 (d, 1H, C5–H, J = 3.5 Hz), 4.97 (d, 1H, C10b–H, J = 8 Hz), 6.54 (s, 1H, C3′–H), 6.80–6.84 (m, 2H, C8–H and C10–H), 7.02 (t, 1H, C9–H, J = 8 Hz), 7.18 (d, 1H, C6′–H, J = 8 Hz), 7.28 (s, 1H, C8′–H), 7.88 (bs, 1H, C5′–H); MS = 378.6 (M + H); anal. calcd for C23H23NO4 (377.43): C, 73.19; H, 6.14; N, 3.71%. Found: C, 73.09; H, 6.22; N, 3.63%.
C); 1299, 1266, 1187, 1134, 1085, 865, 822 cm−1; 1H NMR (500 MHz, CDCl3): δ 1.57–1.60 (m, 1H, C3a–H), 1.72–1.82 (m, 3H, C3b–H and C4–H), 2.49 (bs, 1H, C4a–H), 2.54 (s, 3H, CH3), 3.76 (td, 1H, C2a–H, J = 2.5 and 10.5 Hz), 3.90 (s, 3H, OCH3), 4.00 (bd, 1H, C2b–H), 4.25 (bs, 1H, NH), 4.62 (d, 1H, C5–H, J = 3.5 Hz), 4.97 (d, 1H, C10b–H, J = 8 Hz), 6.54 (s, 1H, C3′–H), 6.80–6.84 (m, 2H, C8–H and C10–H), 7.02 (t, 1H, C9–H, J = 8 Hz), 7.18 (d, 1H, C6′–H, J = 8 Hz), 7.28 (s, 1H, C8′–H), 7.88 (bs, 1H, C5′–H); MS = 378.6 (M + H); anal. calcd for C23H23NO4 (377.43): C, 73.19; H, 6.14; N, 3.71%. Found: C, 73.09; H, 6.22; N, 3.63%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1617, 1585 and 1554 (aromatic C
O); 1617, 1585 and 1554 (aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1487, 1326, 1244, 1179, 1135, 1089, 861, 744 cm−1; 1H NMR (500 MHz, CDCl3): δ 1.43 (bd, 1H, C4–H), 1.55–1.66 (m, 3H, C3–H and C4b–H), 2.37 (s, 3H, CH3), 2.46–2.49 (m, 1H, C4a–H), 2.55 (s, 3H, CH3), 3.52 (td, 1H, C2a–H, J = 2.5 and 10.5 Hz), 3.68 (m, 2H, C2b–H and NH), 5.07 (s, 1H, C5–H), 5.46 (d, 1H, C10b–H, J = 5.5 Hz), 6.69 (d 1H, C7–H, J = 8 Hz), 6.80 (s, 1H, C3′–H), 7.03 (d, 1H, C8–H, J = 8 Hz), 7.21 (d, 1H, C6′–H, J = 8 Hz), 7.28 (s, 1H, C10–H), 7.35 (s, 1H, C8′–H), 7.53 (d, 1H, C5′–H, J = 8 Hz). 13C NMR (75 MHz, CDCl3): 18.5 (C3), 20.7 (CH3), 21.6 (CH3), 25.27 (C4), 35.9 (C4a), 54.5 (C5), 60.6 (C2), 72.3 (C10b), 112.3 (C3′), 115.0 (C4a′), 115.4 (C8′), 117.9 (C7), 119.9 (C10a), 122.9 (C8), 125.7 (C10), 127.8 (C6′), 128.7 (C6a′), 129.1 (C5′), 141.8 (C9), 143.3 (C7′), 154.0 (C4′), 154.1 (C8a′), 161.0 (C2′); MS = 362.5 (M + H). Anal. calcd for C23H23NO3 (361.43): C, 76.43; H, 6.41; N, 3.88%. Found: C, 76.58; H, 6.62; N, 3.92%.
C); 1487, 1326, 1244, 1179, 1135, 1089, 861, 744 cm−1; 1H NMR (500 MHz, CDCl3): δ 1.43 (bd, 1H, C4–H), 1.55–1.66 (m, 3H, C3–H and C4b–H), 2.37 (s, 3H, CH3), 2.46–2.49 (m, 1H, C4a–H), 2.55 (s, 3H, CH3), 3.52 (td, 1H, C2a–H, J = 2.5 and 10.5 Hz), 3.68 (m, 2H, C2b–H and NH), 5.07 (s, 1H, C5–H), 5.46 (d, 1H, C10b–H, J = 5.5 Hz), 6.69 (d 1H, C7–H, J = 8 Hz), 6.80 (s, 1H, C3′–H), 7.03 (d, 1H, C8–H, J = 8 Hz), 7.21 (d, 1H, C6′–H, J = 8 Hz), 7.28 (s, 1H, C10–H), 7.35 (s, 1H, C8′–H), 7.53 (d, 1H, C5′–H, J = 8 Hz). 13C NMR (75 MHz, CDCl3): 18.5 (C3), 20.7 (CH3), 21.6 (CH3), 25.27 (C4), 35.9 (C4a), 54.5 (C5), 60.6 (C2), 72.3 (C10b), 112.3 (C3′), 115.0 (C4a′), 115.4 (C8′), 117.9 (C7), 119.9 (C10a), 122.9 (C8), 125.7 (C10), 127.8 (C6′), 128.7 (C6a′), 129.1 (C5′), 141.8 (C9), 143.3 (C7′), 154.0 (C4′), 154.1 (C8a′), 161.0 (C2′); MS = 362.5 (M + H). Anal. calcd for C23H23NO3 (361.43): C, 76.43; H, 6.41; N, 3.88%. Found: C, 76.58; H, 6.62; N, 3.92%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1620, 1586, 1557 and 1499 (aromatic C
O); 1620, 1586, 1557 and 1499 (aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1447, 1313, 1244, 1141, 1082, 822, 749 cm−1; 1H NMR (500 MHz, CDCl3): δ 1.53–1.58 (m, 1H, C3a–H), 1.70–1.88 (m, 3H, C3b–H and C4–H), 2.35 (s, 3H, CH3), 2.47 (bs, 1H, C4a–H), 2.54 (s, 3H, CH3) 3.77 (td, 1H, C2a–H, J = 2.5 and 10.5 Hz), 4.05 (bd, 1H, C2b–H), 4.52 (bs, 1H, NH), 4.54 (d, 1H, C5–H, J = 3 Hz), 4.97 (d, 1H, C10b–H, J = 8.5 Hz), 6.55 (s, 1H, C3′–H), 6.61 (d, 1H, C7–H, J = 8.0), 7.04 (d, 1H, C8–H, J = 8.0 Hz), 7.16–7.18 (m, 2H, C10–H and C6′–H), 7.27 (s, 1H, C8′–H), 7.93 (bs, 1H, C5′–H). 13C NMR (75 MHz, CDCl3): δ 21.7 (2CH3), 23.1 (C3), 24.5 (C4), 36.6 (C4a), 55.6 (C5), 60.1 (C2), 72.1 (C10b), 109.3 (C3′), 113.8 (C8′), 115.5 (C4a′), 117.3 (C7), 117.8 (C8), 119.4 (C10a), 121.6 (C10), 124.7 (C6′), 125.5 (C5′), 133.3 (C6a), 143.3 (C7′), 146.0 (C9), 154.3 (C4′), 156.2 (C8a′), 161.2 (C2′); MS = 362.5 (M + H); anal. Calcd for C23H23NO3 (361.43): C, 76.43; H, 6.41; N, 3.88%. Found: C, 76.63; H, 6.33; N, 3.75%.
C); 1447, 1313, 1244, 1141, 1082, 822, 749 cm−1; 1H NMR (500 MHz, CDCl3): δ 1.53–1.58 (m, 1H, C3a–H), 1.70–1.88 (m, 3H, C3b–H and C4–H), 2.35 (s, 3H, CH3), 2.47 (bs, 1H, C4a–H), 2.54 (s, 3H, CH3) 3.77 (td, 1H, C2a–H, J = 2.5 and 10.5 Hz), 4.05 (bd, 1H, C2b–H), 4.52 (bs, 1H, NH), 4.54 (d, 1H, C5–H, J = 3 Hz), 4.97 (d, 1H, C10b–H, J = 8.5 Hz), 6.55 (s, 1H, C3′–H), 6.61 (d, 1H, C7–H, J = 8.0), 7.04 (d, 1H, C8–H, J = 8.0 Hz), 7.16–7.18 (m, 2H, C10–H and C6′–H), 7.27 (s, 1H, C8′–H), 7.93 (bs, 1H, C5′–H). 13C NMR (75 MHz, CDCl3): δ 21.7 (2CH3), 23.1 (C3), 24.5 (C4), 36.6 (C4a), 55.6 (C5), 60.1 (C2), 72.1 (C10b), 109.3 (C3′), 113.8 (C8′), 115.5 (C4a′), 117.3 (C7), 117.8 (C8), 119.4 (C10a), 121.6 (C10), 124.7 (C6′), 125.5 (C5′), 133.3 (C6a), 143.3 (C7′), 146.0 (C9), 154.3 (C4′), 156.2 (C8a′), 161.2 (C2′); MS = 362.5 (M + H); anal. Calcd for C23H23NO3 (361.43): C, 76.43; H, 6.41; N, 3.88%. Found: C, 76.63; H, 6.33; N, 3.75%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1615, 1599 and 1500 (aromatic C
O); 1615, 1599 and 1500 (aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1322, 1265, 1087, 1039, 871, 810 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.25–1.65 (m, 4H, C3–H and C4–H), 2.39–2.41 (bd, 1H, C4a–H), 2.46 (s, 3H, CH3), 3.41 (td, 1H, C2a–H, J = 2.5 and 10.0 Hz), 3.60 (dd, 1H, C2b–H, J = 2.5 and 10.0 Hz), 3.89 (s, 1H, NH), 5.00 (s, 1H, C5–H), 5.34 (d, 1H, C10b–H, J = 5.7 Hz), 6.59 (d, 1H, C7–H, J = 8.4 Hz), 6.66 (s, 1H, C3′–H), 7.13 (d, 1H, C6′–H, J = 8 Hz), 7.17 (s, 1H, C8′–H) 7.20 (dd, 1H, C8–H, J = 2.4 and 8 Hz), 7.43 (d, 1H, C5′–H, J = 8 Hz), 7.54 (d, 1H, C10–H, J = 2.4 Hz). 13C NMR (75 MHz, CDCl3): δ 18.6 (C3), 21.6 (CH3), 25.1 (C4), 35.3 (C4a), 54.3 (C5), 60.8 (C2), 71.5 (C10b), 111.4 (C4a′), 112.2 (C3′), 114.8 (C9), 117.0 (C8′), 118.0 (C7′), 122.2 (C10a), 122.9 (C6′), 125.8 (C5′), 130.2 (C8), 131.3 (C10), 143.3 (C6a), 143.6 (C7′), 153.7 (C4′), 154.0 (C8a′), 160.8 (C2′); MS = 427.4 (M + H). Anal. calcd for C22H20BrNO3 (426.30): C, 61.98; H, 4.73; N, 3.29%. Found: C, 61.89; H, 4.80; N, 3.43%.
C); 1322, 1265, 1087, 1039, 871, 810 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.25–1.65 (m, 4H, C3–H and C4–H), 2.39–2.41 (bd, 1H, C4a–H), 2.46 (s, 3H, CH3), 3.41 (td, 1H, C2a–H, J = 2.5 and 10.0 Hz), 3.60 (dd, 1H, C2b–H, J = 2.5 and 10.0 Hz), 3.89 (s, 1H, NH), 5.00 (s, 1H, C5–H), 5.34 (d, 1H, C10b–H, J = 5.7 Hz), 6.59 (d, 1H, C7–H, J = 8.4 Hz), 6.66 (s, 1H, C3′–H), 7.13 (d, 1H, C6′–H, J = 8 Hz), 7.17 (s, 1H, C8′–H) 7.20 (dd, 1H, C8–H, J = 2.4 and 8 Hz), 7.43 (d, 1H, C5′–H, J = 8 Hz), 7.54 (d, 1H, C10–H, J = 2.4 Hz). 13C NMR (75 MHz, CDCl3): δ 18.6 (C3), 21.6 (CH3), 25.1 (C4), 35.3 (C4a), 54.3 (C5), 60.8 (C2), 71.5 (C10b), 111.4 (C4a′), 112.2 (C3′), 114.8 (C9), 117.0 (C8′), 118.0 (C7′), 122.2 (C10a), 122.9 (C6′), 125.8 (C5′), 130.2 (C8), 131.3 (C10), 143.3 (C6a), 143.6 (C7′), 153.7 (C4′), 154.0 (C8a′), 160.8 (C2′); MS = 427.4 (M + H). Anal. calcd for C22H20BrNO3 (426.30): C, 61.98; H, 4.73; N, 3.29%. Found: C, 61.89; H, 4.80; N, 3.43%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1617, 1600, 1513 and 1484 (aromatic C
O); 1617, 1600, 1513 and 1484 (aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1303, 1271, 1133, 1087, 806 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.49–1.86 (m, 4H, C3–H and C4–H), 2.35 (bs, 1H, C4a–H), 2.45 (s, 3H, CH3), 3.63–3.70 (m, 1H, C2a–H), 3.85–3.89 (m, 1H, C2b–H), 4.27 (s, 1H, NH), 4.46 (d, 1H, C5–H, J = 3.6 Hz), 4.83 (d, 1H, C10b–H, J = 8 Hz), 6.38 (s, 1H, C3′–H), 6.54 (d, 1H, C7–H, J = 8.4), 7.10 (d, 1H, C6′–H), 7.16 (s, 1H, C8′–H), 7.23 (dd, 1H, C8–H, 2.1 and 8.7 Hz), 7.40 (d, 1H, C10–H, J = 2.1 Hz), 7.70 (d, 1H, C5′–H, J = 8.1 Hz). 13C NMR (75 MHz, CDCl3): 21.7 (CH3), 23.8 (C3), 24.3 (C4), 36.1 (C4a), 55.6 (C5), 60.7 (C2), 71.5 (C10b), 109.9 (C4a′), 113.7 (C3′), 115.2 (C9), 115.8 (C8′), 117.9 (C7′), 121.3 (C10a), 124.2 (C6′), 125.6 (C5′), 132.1 (C8), 132.3 (C10), 142.2 (C6a), 143.5 (C7′), 154.2 (C4′), 155.6 (C8a′), 161.0 (C2′); MS = 427.4 (M + H); anal. calcd for C22H20BrNO3 (426.30): C, 61.98; H, 4.73; N, 3.29%. Found: C, 61.87; H, 4.76; N, 3.44%.
C); 1303, 1271, 1133, 1087, 806 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.49–1.86 (m, 4H, C3–H and C4–H), 2.35 (bs, 1H, C4a–H), 2.45 (s, 3H, CH3), 3.63–3.70 (m, 1H, C2a–H), 3.85–3.89 (m, 1H, C2b–H), 4.27 (s, 1H, NH), 4.46 (d, 1H, C5–H, J = 3.6 Hz), 4.83 (d, 1H, C10b–H, J = 8 Hz), 6.38 (s, 1H, C3′–H), 6.54 (d, 1H, C7–H, J = 8.4), 7.10 (d, 1H, C6′–H), 7.16 (s, 1H, C8′–H), 7.23 (dd, 1H, C8–H, 2.1 and 8.7 Hz), 7.40 (d, 1H, C10–H, J = 2.1 Hz), 7.70 (d, 1H, C5′–H, J = 8.1 Hz). 13C NMR (75 MHz, CDCl3): 21.7 (CH3), 23.8 (C3), 24.3 (C4), 36.1 (C4a), 55.6 (C5), 60.7 (C2), 71.5 (C10b), 109.9 (C4a′), 113.7 (C3′), 115.2 (C9), 115.8 (C8′), 117.9 (C7′), 121.3 (C10a), 124.2 (C6′), 125.6 (C5′), 132.1 (C8), 132.3 (C10), 142.2 (C6a), 143.5 (C7′), 154.2 (C4′), 155.6 (C8a′), 161.0 (C2′); MS = 427.4 (M + H); anal. calcd for C22H20BrNO3 (426.30): C, 61.98; H, 4.73; N, 3.29%. Found: C, 61.87; H, 4.76; N, 3.44%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1612, 1587 and 1497 (aromatic C
O); 1612, 1587 and 1497 (aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1552 and 1365 (NO2), 1316, 1267, 1132, 1089, 822, 748 cm−1; 1H NMR (300 MHz, CDCl3 + DMSO): δ 1.25–1.53 (m, 4H, C3–H and C4–H), 2.48 (s, 3H, CH3), 2.59 (bt, 1H, C4a–H, merged in DMSO), 2.89 (bs, 1H, NH, merged in shifted DMSO water), 3.41 (td, 1H, C2a–H, J = 2.5 and 10.5 Hz), 3.65 (dd, 1H, C2b–H, J = 2.5 and 10.5 Hz), 5.18 (s, 1H, C5–H), 5.38 (d, 1H, C10b–H, J = 5.7 Hz), 6.61 (s, 1H, C3′–H), 6.86 (d, 1H, C7–H, J = 9.3 Hz), 7.18 (d, 1H, C6′–H, J = 8 Hz), 7.20 (s, 1H, C8′–H) 7.54 (d, 1H, C5′–H, J = 8 Hz), 7.96 (dd, 1H, C8–H, J = 2.7 and 9.3 Hz), 8.26 (d, 1H, C10–H, J = 2.4 Hz). MS = 393.3 (M + H); anal. calcd for C22H20N2O5 (392.40): C, 67.34; H, 5.14; N, 7.14%. Found: C, 67.47; H, 5.08; N, 7.06%.
C); 1552 and 1365 (NO2), 1316, 1267, 1132, 1089, 822, 748 cm−1; 1H NMR (300 MHz, CDCl3 + DMSO): δ 1.25–1.53 (m, 4H, C3–H and C4–H), 2.48 (s, 3H, CH3), 2.59 (bt, 1H, C4a–H, merged in DMSO), 2.89 (bs, 1H, NH, merged in shifted DMSO water), 3.41 (td, 1H, C2a–H, J = 2.5 and 10.5 Hz), 3.65 (dd, 1H, C2b–H, J = 2.5 and 10.5 Hz), 5.18 (s, 1H, C5–H), 5.38 (d, 1H, C10b–H, J = 5.7 Hz), 6.61 (s, 1H, C3′–H), 6.86 (d, 1H, C7–H, J = 9.3 Hz), 7.18 (d, 1H, C6′–H, J = 8 Hz), 7.20 (s, 1H, C8′–H) 7.54 (d, 1H, C5′–H, J = 8 Hz), 7.96 (dd, 1H, C8–H, J = 2.7 and 9.3 Hz), 8.26 (d, 1H, C10–H, J = 2.4 Hz). MS = 393.3 (M + H); anal. calcd for C22H20N2O5 (392.40): C, 67.34; H, 5.14; N, 7.14%. Found: C, 67.47; H, 5.08; N, 7.06%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1612, 1528 and 1484 (aromatic C
O); 1612, 1528 and 1484 (aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1577 and 1377 (NO2), 1334, 1271, 1136, 1079, 798, 723 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.25–1.58 (m, 4H, C3–H and C4–H), 2.42–2.47 (m, 4H, CH3 and C4a–H), 3.09–3.14 (m, 1H, C2a–H), 3.57 (d, 1H, C2b–H, J = 10.5 Hz), 4.19 (s, 1H, NH), 5.02 (s, 1H, C5–H), 5.70 (d, 1H, C10b–H, J = 6.0 Hz), 6.63 (s, 1H, C3′–H), 6.88 (d, 1H, C7–H, J = 8.4 Hz), 6.98 (d, 1H, C9–H, J = 7.8 Hz) 7.15–7.44 (m, 4H, C8–H, C6′–H, C8′–H and C5′–H); 13C NMR (75 MHz, CDCl3): δ 17.7 (C3), 20.8 (CH3), 23.8 (C4), 33.7 (C4a), 53.0 (C5), 60.9 (C2), 68.6 (C10b), 111.4 (C4), 111.7 (C3′), 111.8 (C8′), 114.0 (C4a′), 116.9 (C7), 117.7 (C8), 122.5, 125.1, 128.0 (C9), 142.7 (C7′), 146.5 (C6a), 150.5 (C10), 152.4 (C8a′), 153.1 (C4′), 160.0 (C2′); MS = 393.3 (M + H); anal. calcd for C22H20N2O5 (392.40): C, 67.34; H, 5.14; N, 7.14%. Found: C, 67.22; H, 5.02; N, 7.00%.
C); 1577 and 1377 (NO2), 1334, 1271, 1136, 1079, 798, 723 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.25–1.58 (m, 4H, C3–H and C4–H), 2.42–2.47 (m, 4H, CH3 and C4a–H), 3.09–3.14 (m, 1H, C2a–H), 3.57 (d, 1H, C2b–H, J = 10.5 Hz), 4.19 (s, 1H, NH), 5.02 (s, 1H, C5–H), 5.70 (d, 1H, C10b–H, J = 6.0 Hz), 6.63 (s, 1H, C3′–H), 6.88 (d, 1H, C7–H, J = 8.4 Hz), 6.98 (d, 1H, C9–H, J = 7.8 Hz) 7.15–7.44 (m, 4H, C8–H, C6′–H, C8′–H and C5′–H); 13C NMR (75 MHz, CDCl3): δ 17.7 (C3), 20.8 (CH3), 23.8 (C4), 33.7 (C4a), 53.0 (C5), 60.9 (C2), 68.6 (C10b), 111.4 (C4), 111.7 (C3′), 111.8 (C8′), 114.0 (C4a′), 116.9 (C7), 117.7 (C8), 122.5, 125.1, 128.0 (C9), 142.7 (C7′), 146.5 (C6a), 150.5 (C10), 152.4 (C8a′), 153.1 (C4′), 160.0 (C2′); MS = 393.3 (M + H); anal. calcd for C22H20N2O5 (392.40): C, 67.34; H, 5.14; N, 7.14%. Found: C, 67.22; H, 5.02; N, 7.00%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1617, 1589 and 1496 (aromatic C
O); 1617, 1589 and 1496 (aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1528 and 1361 (NO2), 1319, 1270, 1134, 1079, 824, 720 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.4–1.9 (m, 4H, C3–H and C4–H), 2.46–2.47 (m, 4H, CH3 and C4a–H), 3.64–3.99 (m, 2H, C2a–H and C2b–H), 4.46 (bs, 1H, NH), 4.58 (d, 1H, C5–H, J = 3.6 Hz), 5.13 (d, 1H, C10b–H, J = 8 Hz), 6.37 (s, 1H, C3′–H), 6.80 (t, 1H, C8–H, J = 8.0), 7.13 (d, 1H, C6′–H, J = 8.1 Hz), 7.22 (m, 2H, C7–H and C8′–H, J = 2.1 and 9 Hz), 7.60 (dd, 1H, C9–H, 2.1 and 8.0 Hz), 7.64 (d, 1H, C5′–H, J = 8.1 Hz); MS = 393.5 (M + H); anal. calcd for C22H20N2O5 (392.40): C, 67.34; H, 5.14; N, 7.14%. Found: C, 67.17; H, 5.10; N, 7.23%.
C); 1528 and 1361 (NO2), 1319, 1270, 1134, 1079, 824, 720 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.4–1.9 (m, 4H, C3–H and C4–H), 2.46–2.47 (m, 4H, CH3 and C4a–H), 3.64–3.99 (m, 2H, C2a–H and C2b–H), 4.46 (bs, 1H, NH), 4.58 (d, 1H, C5–H, J = 3.6 Hz), 5.13 (d, 1H, C10b–H, J = 8 Hz), 6.37 (s, 1H, C3′–H), 6.80 (t, 1H, C8–H, J = 8.0), 7.13 (d, 1H, C6′–H, J = 8.1 Hz), 7.22 (m, 2H, C7–H and C8′–H, J = 2.1 and 9 Hz), 7.60 (dd, 1H, C9–H, 2.1 and 8.0 Hz), 7.64 (d, 1H, C5′–H, J = 8.1 Hz); MS = 393.5 (M + H); anal. calcd for C22H20N2O5 (392.40): C, 67.34; H, 5.14; N, 7.14%. Found: C, 67.17; H, 5.10; N, 7.23%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1619, 1550 and 1501 (aromatic C
O); 1619, 1550 and 1501 (aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1443, 1320, 1262, 1232, 1147, 1067, 862, 817 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.48–1.83 (m, 4H, C3–H and C4–H), 2.38–2.39 (bd, 1H, C4a–H), 2.45 (s, 3H, CH3), 3.64–3.72 (m, 1H, C2a–H), 3.77 (s, 3H, OCH3), 3.92 (m, 2H, NH, C2b–H), 4.48 (d, 1H, C5–H, J = 3.6 Hz), 4.85 (d, 1H, C10b–H, J = 8 Hz), 6.46 (s, 1H, C3′–H), 6.56 (d, 1H, C7–H, J = 8.4), 6.77 (dd, 1H, C8–H, J = 2.7 and 8.4 Hz), 6.87 (d, 1H, C10–H, J = 2.7 Hz), 7.09 (d, 1H, C6′–H, J = 8 Hz), 7.19 (s, 1H, C8′–H), 7.81 (d, 1H, C5′–H, J = 8 Hz); 13C NMR (75 MHz, CDCl3): δ 18.5 (C3), 21.6 (CH3), 25.2 (C4), 35.8 (C4a), 54.6 (OCH3), 55.9 (C5), 60.8 (C2), 72.4 (C10b), 111.8 (C3), 112.3 (C8), 115.0 (C4a′), 115.4 (C9), 116.6 (C7′), 117.9 (C8′), 121.2 (C10a), 122.9 (C6′), 125.6 (C5′), 138.1 (C6a), 143.3 (C7′), 153.5 (C9), 154.0 (C4′), 154.2 (C8a′), 161.0 (C2′); MS = 378.4 (M + H); anal. calcd for C23H23NO4 (377.43): C, 73.19; H, 6.14; N, 3.71%. Found: C, 73.34; H, 6.21; N, 3.66%.
C); 1443, 1320, 1262, 1232, 1147, 1067, 862, 817 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.48–1.83 (m, 4H, C3–H and C4–H), 2.38–2.39 (bd, 1H, C4a–H), 2.45 (s, 3H, CH3), 3.64–3.72 (m, 1H, C2a–H), 3.77 (s, 3H, OCH3), 3.92 (m, 2H, NH, C2b–H), 4.48 (d, 1H, C5–H, J = 3.6 Hz), 4.85 (d, 1H, C10b–H, J = 8 Hz), 6.46 (s, 1H, C3′–H), 6.56 (d, 1H, C7–H, J = 8.4), 6.77 (dd, 1H, C8–H, J = 2.7 and 8.4 Hz), 6.87 (d, 1H, C10–H, J = 2.7 Hz), 7.09 (d, 1H, C6′–H, J = 8 Hz), 7.19 (s, 1H, C8′–H), 7.81 (d, 1H, C5′–H, J = 8 Hz); 13C NMR (75 MHz, CDCl3): δ 18.5 (C3), 21.6 (CH3), 25.2 (C4), 35.8 (C4a), 54.6 (OCH3), 55.9 (C5), 60.8 (C2), 72.4 (C10b), 111.8 (C3), 112.3 (C8), 115.0 (C4a′), 115.4 (C9), 116.6 (C7′), 117.9 (C8′), 121.2 (C10a), 122.9 (C6′), 125.6 (C5′), 138.1 (C6a), 143.3 (C7′), 153.5 (C9), 154.0 (C4′), 154.2 (C8a′), 161.0 (C2′); MS = 378.4 (M + H); anal. calcd for C23H23NO4 (377.43): C, 73.19; H, 6.14; N, 3.71%. Found: C, 73.34; H, 6.21; N, 3.66%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1611, and 1501 (aromatic C
O); 1611, and 1501 (aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1442, 1366, 1230, 1218, 1158, 1077, 1038, 921, 821 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.34–1.60 (m, 4H, C3–H and C4–H), 2.39 (bs, 1H, C4a–H) 2.46 (s, 3H, CH3) 3.42 (td, 1H, C2a–H), 3.53 (s, 1H, NH) 3.61 (dd, 1H, C2b–H), 3.79 (s, 3H, OCH3) 4.96 (s, 1H, C5–H), 5.38 (d, 1H, C10b–H, J = 5.5 Hz), 6.64 (d 1H, C7–H, J = 9.0 Hz), 6.73 (s, 1H, C3′–H), 6.76 (dd, 1H, C8–H, J = 2.7 and 8 Hz), 7.03 (d, 1H, C10–H), 7.13 (d, 1H, C6′–H, J = 8 Hz), 7.19 (s, 1H, C8′–H), 7.45 (d, 1H, C5′–H, J = 8 Hz); MS = 378.6 (M + H); anal. calcd for C23H23NO4 (377.43): C, 73.19; H, 6.14; N, 3.71%. Found: C, 73.29; H, 6.23; N, 3.79%.
C); 1442, 1366, 1230, 1218, 1158, 1077, 1038, 921, 821 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.34–1.60 (m, 4H, C3–H and C4–H), 2.39 (bs, 1H, C4a–H) 2.46 (s, 3H, CH3) 3.42 (td, 1H, C2a–H), 3.53 (s, 1H, NH) 3.61 (dd, 1H, C2b–H), 3.79 (s, 3H, OCH3) 4.96 (s, 1H, C5–H), 5.38 (d, 1H, C10b–H, J = 5.5 Hz), 6.64 (d 1H, C7–H, J = 9.0 Hz), 6.73 (s, 1H, C3′–H), 6.76 (dd, 1H, C8–H, J = 2.7 and 8 Hz), 7.03 (d, 1H, C10–H), 7.13 (d, 1H, C6′–H, J = 8 Hz), 7.19 (s, 1H, C8′–H), 7.45 (d, 1H, C5′–H, J = 8 Hz); MS = 378.6 (M + H); anal. calcd for C23H23NO4 (377.43): C, 73.19; H, 6.14; N, 3.71%. Found: C, 73.29; H, 6.23; N, 3.79%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1608, 1591 and 1485 (aromatic C
O); 1608, 1591 and 1485 (aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1362, 1261, 1187, 1151, 872, 829, 750 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.76–1.83 (m, 1H, C3a–H) 2.1–2.25 (m, 1H, C3b–H), 2.46 (s, 3H, CH3), 2.64–2.74 (bm, 1H, C3a–H), 3.87 (m, 1H, C2a–H), 4.03 (m, 1H, C2b–H), 4.17 (bs, 1H, NH), 4.23 (d, 1H, C4–H, J = 10.2 Hz), 4.65 (d, 1H, C9a–H, J = 5.7 Hz), 6.55 (s, 1H, C3′–H), 6.67 (d, 1H, C6–H, J = 7.5 Hz), 6.87 (t, 1H, C8–H, J = 2 and 8 Hz), 7.10 (d, 1H, C6′–H, J = 8.1 Hz), 7.17 (dd, 1H, C7–H, J = 8), 7.21 (s, 1H, C8′–H), 7.42 (d, 1H, C9–H, J = 7.8 Hz), 7.85 (d, 1H, C5′–H, J = 7.8 Hz); MS = 334.5 (M + H); anal. calcd for C21H19NO3 (333.38): C, 75.66; H, 5.74; N, 4.20%. Found: C, 75.78; H, 5.85; N, 4.12%.
C); 1362, 1261, 1187, 1151, 872, 829, 750 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.76–1.83 (m, 1H, C3a–H) 2.1–2.25 (m, 1H, C3b–H), 2.46 (s, 3H, CH3), 2.64–2.74 (bm, 1H, C3a–H), 3.87 (m, 1H, C2a–H), 4.03 (m, 1H, C2b–H), 4.17 (bs, 1H, NH), 4.23 (d, 1H, C4–H, J = 10.2 Hz), 4.65 (d, 1H, C9a–H, J = 5.7 Hz), 6.55 (s, 1H, C3′–H), 6.67 (d, 1H, C6–H, J = 7.5 Hz), 6.87 (t, 1H, C8–H, J = 2 and 8 Hz), 7.10 (d, 1H, C6′–H, J = 8.1 Hz), 7.17 (dd, 1H, C7–H, J = 8), 7.21 (s, 1H, C8′–H), 7.42 (d, 1H, C9–H, J = 7.8 Hz), 7.85 (d, 1H, C5′–H, J = 7.8 Hz); MS = 334.5 (M + H); anal. calcd for C21H19NO3 (333.38): C, 75.66; H, 5.74; N, 4.20%. Found: C, 75.78; H, 5.85; N, 4.12%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1615, 1555 and 1486 (aromatic C
O); 1615, 1555 and 1486 (aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1321, 1255, 1186, 1061, 867, 772, 701, 755 cm−1; 1H NMR (400 MHz, DMSO): δ 1.96–2.02 (m, 1H, C3a–H) 2.39 (d, 1H, C3b–H, J = 12 Hz), 2.41 (s, 3H, CH3), 4.44 (dd, 1H, C4–H, J = 5 and 12 Hz), 5.08 (d, 1H, C2–H, J = 8.8 Hz), 6.17 (s, 1H, C3′–H), 6.39–6.47 (m, 2H, C6–H and C8–H, J = 8 Hz), 6.58 (s, 1H, C8′–H) 6.74 (d, 1H, C5–H, J = 7.8 Hz), 6.95 (t, 1H, PhH, J = 7 Hz), 7.19–7.33 (m, 6H, C6′–H, C7–H, 4PhH), 7.92 (d, 1H, C5′–H, J = 8.3 Hz); MS = 368.3 (M + H). Anal. calcd for C25H21NO2 (367.44): C, 81.72; H, 5.76; N, 3.81%. Found: C, 81.64; H, 5.85; N, 3.74%.
C); 1321, 1255, 1186, 1061, 867, 772, 701, 755 cm−1; 1H NMR (400 MHz, DMSO): δ 1.96–2.02 (m, 1H, C3a–H) 2.39 (d, 1H, C3b–H, J = 12 Hz), 2.41 (s, 3H, CH3), 4.44 (dd, 1H, C4–H, J = 5 and 12 Hz), 5.08 (d, 1H, C2–H, J = 8.8 Hz), 6.17 (s, 1H, C3′–H), 6.39–6.47 (m, 2H, C6–H and C8–H, J = 8 Hz), 6.58 (s, 1H, C8′–H) 6.74 (d, 1H, C5–H, J = 7.8 Hz), 6.95 (t, 1H, PhH, J = 7 Hz), 7.19–7.33 (m, 6H, C6′–H, C7–H, 4PhH), 7.92 (d, 1H, C5′–H, J = 8.3 Hz); MS = 368.3 (M + H). Anal. calcd for C25H21NO2 (367.44): C, 81.72; H, 5.76; N, 3.81%. Found: C, 81.64; H, 5.85; N, 3.74%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1615, 1554 and 1503 (aromatic C
O); 1615, 1554 and 1503 (aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1321, 1259, 1186, 1146, 870, 755 cm−1; 1H NMR (400 MHz, DMSO): δ 1.83–1.90 (bs, 1H, C3a–H) 2.35 (bs, 1H, C3b–H), 2.40 (s, 3H, CH3), 4.35–4.43 (m, 1H, C4–H), 5.04 (bs, 1H, NH), 5.39–5.41 (m, 1H, C2–H), 6.54 (s, 1H, C3′–H), 6.67–6.69 (m, 2H, ArH), 6.95–7.22 (m, 9H, ArH), 7.90 (d, 1H, C5′–H, J = 8.0 Hz); MS = 368.4 (M + H); anal. calcd for C25H21NO2 (367.44): C, 81.59; H, 5.76; N, 3.81%. Found: C, 81.59; H, 5.82; N, 3.75%.
C); 1321, 1259, 1186, 1146, 870, 755 cm−1; 1H NMR (400 MHz, DMSO): δ 1.83–1.90 (bs, 1H, C3a–H) 2.35 (bs, 1H, C3b–H), 2.40 (s, 3H, CH3), 4.35–4.43 (m, 1H, C4–H), 5.04 (bs, 1H, NH), 5.39–5.41 (m, 1H, C2–H), 6.54 (s, 1H, C3′–H), 6.67–6.69 (m, 2H, ArH), 6.95–7.22 (m, 9H, ArH), 7.90 (d, 1H, C5′–H, J = 8.0 Hz); MS = 368.4 (M + H); anal. calcd for C25H21NO2 (367.44): C, 81.59; H, 5.76; N, 3.81%. Found: C, 81.59; H, 5.82; N, 3.75%.Conclusions
In conclusion, the reaction of 7-methylcoumarin-4-carbaldehyde with substituted anilines gives 7-methylcoumarin-4-azadienes (1). These azadienes provide two azadiene components, one involving coumarin 3,4-double bond (N) and the other involving the aniline ring (M). The two dienes differ markedly in reactivity. Compound 1 does not undergo normal electron demand Diels–Alder reaction similar to 4-styrylcoumarin. It undergoes regiospecific inverse electron demand Diels–Alder reaction involving diene M, with dienophiles dihydropyran, dihydrofuran, and styrene. The reaction requires a Lewis acid catalyst and the ZnCl2 is the best catalyst. The solvent has profound effect on the reaction; 1,2-dichloroethane is the best solvent. In the reaction unequal amounts of two diastereomeric products are formed. The reaction provides an entry into coumarinyl substituted pyranoquinolines.Acknowledgements
Authors are thankful to Prof. K.V.R. Chary, TIFR, Mumbai, India for providing 2D NMR facility and for interpretation of the results and Dr Vasuki, G. Department of Physics, Kunthavai Naachiar Government Arts College (W) (Autonomous), Thanjavur-7, India, for providing single crystal X-ray facility.Notes and references
- (a) M. P. S. Ishar, G. Singh, S. Singh, K. K. Sreenivasan and G. Singh, Bioorg. Med. Chem. Lett., 2006, 16, 1366–1370 CrossRef CAS PubMed; (b) J. Nawrot-Modranka, E. Nawrot and J. Graczyk, Eur. J. Med. Chem., 2006, 41, 1301–1309 CrossRef CAS PubMed; (c) P. Valenti, G. Fabbri, A. Rampa, A. Bisi, S. Gobbi, P. Da Re, M. Carrara, A. Sgevano and L. Cima, Anticancer Drug Des., 1996, 11, 243–252 CAS.
- (a) M. Lácová, H. Stankoviĉová and Ž. Odlerová, Farmaco, 1995, 50, 885–888 Search PubMed; (b) H. M. El-Shaaer, P. Foltínová, M. Lácová, J. Chovancová and H. Stankoviĉová, Farmaco, 1998, 53, 224–232 CrossRef CAS; (c) O. Kayser and H. Kolodziej, Z. Naturforsch., 1999, 54c, 169–174 Search PubMed.
- (a) S. Kirkiacharian, D. T. Thuy, S. Sicsic, R. Bakhchinian, R. Kurkijan and T. Tonnaire, Farmaco, 2002, 57, 703–708 CrossRef CAS; (b) P. C. M. Mao, J. F. Mouscadet, H. Leh, C. Auclair and L. Y. Hsu, Chem. Pharm. Bull., 2002, 50, 1634–1637 CrossRef CAS.
- (a) P. G. Maddela, R. G. Narendar, K. Srinu, J. Manjulatha, P. S. Venkata, K. O. Pramod, I. K. Javed and A. Kumar, Synlett, 2010, 947–951 Search PubMed; (b) R. Girotti, A. Marrocchi, L. Minuti, O. Piermatti, F. Pizzo and L. Vaccaro, J. Org. Chem., 2006, 71, 70–74 CrossRef CAS PubMed; (c) E. Ballerini, L. Minuti and O. Piermatti, J. Org. Chem., 2010, 75, 4251–4260 CrossRef CAS PubMed; (d) D. Amantini, F. Fringuelli, O. Piermatti, F. Pizzo and L. Vaccaro, J. Org. Chem., 2003, 68, 9263–9268 CrossRef CAS PubMed; (e) E. J. Michael and A. A. Damian, Org. Lett., 2009, 11, 757–760 CrossRef PubMed; (f) I. Y. Flores-Larios, G. Lizbeth-Lopez, F. J. Martínez-Martínez, J. González, E. V. García-Báez, A. Cruz and I. I. Padilla-Martínez, Molecules, 2010, 15, 1513–1530 CrossRef CAS PubMed; (g) D. Amantini, F. Fringuelli and F. Pizzo, J. Org. Chem., 2002, 67, 7238–7243 CrossRef CAS PubMed.
- (a) M. A. Hassana, S. A. Shibaa, N. S. Harba, M. K. Abou-El-Regal and S. A. El-Metwally, Synth. Commun., 2002, 32, 679–688 CrossRef; (b) R. R. Chada, K. Nayani, J. Kancharla, P. Mrunal and N. Police, Synthesis, 2009, 399–402 Search PubMed.
- (a) Y. Masahide, K. Toyoaki, G. Chitoshi, S. Hiroshi, N. Kenichi, M. Toru and S. Kensuke, Tetrahedron Lett., 1992, 33, 6465–6468 CrossRef; (b) M. Toru, M. Yasuyuki, N. Seigo, K. Shinichiro and Y. Masahiko, J. Org. Chem., 1992, 57, 167–172 CrossRef; (c) A. Mustafa and K. Mohamad, J. Am. Chem. Soc., 1955, 77, 1828–1830 CrossRef CAS; (d) A. Mustafa, K. Mohamad and A. A. Mohmad, J. Am. Chem. Soc., 1956, 78, 4692–4694 CrossRef CAS; (e) A. Y. Soliman, A. F. El-Kafrawy, F. K. Mohamed, H. M. Baker and A. M. Abdel-Gawad, Indian J. Chem., 1991, 30B, 477–481 CAS; (f) A. E. Shafei, A. A. Fadda, I. I. Abdel-Gawad and E. H. E. Youssif, Synth. Commun., 2009, 39, 2954–2972 CrossRef.
- (a) G. J. Bodwell, Z. Pi and I. R. Pottie, Synlett, 1999, 477–480 CrossRef CAS PubMed; (b) I. R. Pottie, P. R. Nandaluru and G. J. Bodwell, Synlett, 2011, 2245–2247 CAS; (c) R. Pottie, P. R. Nandaluru, W. L. Benoit, D. O. Miller, L. N. Dawe and G. J. Bodwell, J. Org. Chem., 2011, 76, 9015–9030 CrossRef PubMed; (d) P. R. Nandaluru and G. J. Bodwell, Org. Lett., 2012, 14, 310–313 CrossRef CAS PubMed.
- (a) A. A. Kudale, J. Kendall, D. O. Miller, J. L. Collins and G. J. Bodwell, J. Org. Chem., 2008, 73, 8437–8447 CrossRef CAS PubMed; (b) A. A. Kudale, D. O. Miller, L. N. Dawea and G. J. Bodwell, Org. Biomol. Chem., 2011, 9, 7196–7206 RSC.
- (a) A. D. Payne, G. Bojase, M. N. Paddon-Row and M. S. Sherburn, Angew. Chem., Int. Ed., 2009, 48, 4836–4839 CrossRef CAS PubMed; (b) T. A. Bradford, A. D. Payne, A. C. Willis, M. N. Paddon-Row and M. S. Sherburn, Org. Lett., 2007, 9, 2007 CrossRef PubMed; (c) C. G. Newton, S. L. Drew, A. L. Lawrence, A. C. Willis, M. N. Paddon-Row and M. S. Sherburn, Nat. Chem., 2015, 7, 82–86 CrossRef CAS PubMed; E. J. Lindeboom, A. C. Willis, M. N. Paddon-Row and M. S. Sherburn, Angew. Chem., Int. Ed., 2014, 53, 5440–5443 Search PubMed; (d) K. K. Sanap and S. D. Samant, Synlett, 2012, 2189–2194 CAS.
- (a) S. Motoki, Y. Matsuo and Y. Terauchi, Bull. Chem. Soc. Jpn., 1990, 63, 284 CrossRef CAS; (b) T. Saito, H. Kimura, K. Sakamaki, T. Karakasa and S. Moriyama, Chem. Commun., 1996, 811 RSC.
- (a) O. Tsuge, T. Hatta, H. Yoshitomi, K. Kurosaka, T. Fujiwara, H. Maeda and A. Kakehi, Heterocycles, 1995, 41, 225 CrossRef CAS; (b) O. Tsuge, T. Hatta, T. Fujiwara, T. Yokohari and A. Tsuge, Heterocycles, 1999, 50, 661 CrossRef CAS PubMed.
- (a) S. Kobayashi, T. Semba, T. Takahashi, S. Yoshida, K. Dai, T. Otani and T. Saito, Tetrahedron, 2009, 65, 920 CrossRef CAS PubMed; (b) S. Kobayashi, T. Furuya, T. Otani and T. Saito, Tetrahedron Lett., 2008, 49, 4513 CrossRef CAS PubMed; (c) S. Kobayashi, T. Furuya, T. Otani and T. Saito, Tetrahedron, 2008, 64, 9705 CrossRef CAS PubMed; (d) Z. Jin, R. Yang, Y. Du, B. Tiwari, R. Ganguly and Y. R. Chi, Org. Lett., 2012, 14, 3226 CrossRef CAS PubMed; (e) T. Saito, H. Kimura, T. Chonan, T. Soda and T. Karakasa, Chem. Commun., 1997, 1013–1014 RSC.
- S. Kobayashi, K. Kudo, A. Ito, S. Hirama, T. Otani and T. Saito, Org. Biomol. Chem., 2014, 12, 406 Search PubMed.
- J. Sauer and J. Sustmann, Angew. Chem., Int. Ed., 1980, 19, 779–807 CrossRef PubMed.
- (a) P. Buonora, J.-C. Olsen and T. Oh, Tetrahedron, 2001, 57, 6099–6138 CrossRef CAS; (b) V. Kouznetsov, Tetrahedron, 2009, 65, 2721–2750 CrossRef CAS PubMed.
- S. C. Laskowski and R. O. Clinton, J. Am. Chem. Soc., 1950, 72, 3987–3991 CrossRef CAS.
- U. C. Mashelkar and A. A. Audi, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2006, 45, 1463–1469 Search PubMed.
- K. K. Sanap, R. S. Kulkarni and S. D. Samant, J. Heterocycl. Chem., 2013, 50, 713–719 CrossRef CAS PubMed.
- (a) Y.-S. Cheng, E. Ho, P. S. Mariano and H. L. Ammon, J. Org. Chem., 1985, 50, 5678–5686 CrossRef CAS; (b) V. Lucchini, M. Prato, G. Scorrano, M. Stivanel and G. Valle, J. Chem. Soc., Perkin Trans. 2, 1992, 2, 259–266 RSC; (c) M. J. Alves, N. G. Azoia and A. G. Fortes, Tetrahedron, 2007, 63, 727–734 CrossRef CAS PubMed; (d) R. Annunziata, M. Cinquini, F. Cozzi, V. Molteni and O. Schupp, Tetrahedron, 1997, 53, 9715 CrossRef CAS; (e) V. Sridharan, C. Avendao and J. C. MeneÂdez, Tetrahedron, 2007, 63, 673 CrossRef CAS PubMed.
- L. Simón and J. M. Goodman, J. Org. Chem., 2011, 76, 1775 CrossRef PubMed.
- V. A. Glushkov and A. G. Tolstikov, Russ. Chem. Rev., 2008, 77, 137–159 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 901323–901324. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra06262d |
| This journal is © The Royal Society of Chemistry 2015 |