Link spacer controlled supramolecular chirality of perylene bisimide-carbohydrate conjugate†
Ke-Rang Wang*ab,
Dan Hana,
Guo-Jing Caoa and
Xiao-Liu Li*ab
aKey Laboratory of Chemical Biology of Hebei Province, College of Chemistry and Environmental Science, Hebei University, Baoding 071002, China. E-mail: kerangwang@hbu.edu.cn; lixl@hbu.cn
bKey Laboratory of Medicinal Chemistry and Molecular Diagnosis of Ministry of Education, Baoding 071002, China
First published on 18th May 2015
Abstract
Controllable supramolecular chirality based on the self-assembly of the perylene bisimide-carbohydrate conjugates was achieved, which exhibited right-handed chirality under the triazole as the linker, and left-handed chirality with the amide bond as the linker, because of the difference of the chirality transfer induced by the different π⋯π stacking interactions and the additional hydrogen-bonds of the amide bond.
In nature, helical self-assembly by non-covalent interactions is a widely observed feature, for example, the DNA double helix, the α-helices of proteins and polysaccharides, and the helix of (bacterio)chlorophyll arrays.1 Inspired by the unique features of fascinating biological superstructures, chemists have paid more attention to design numerous helical supramolecular assemblies by utilizing non-covalent forces such as hydrogen bonding, π⋯π stacking interaction, and van der Waals forces.2 In this regard, the construction of chiral supramolecular assemblies by π-conjugated systems attracted more interest because of their potential applications in biological systems, electronics and photonics.3 Among them, perylene bisimides (PBIs) have attracted special interest for the well-defined supramolecular self-assemblies, especially for chiral assemblies.4 The controlled chiral assemblies of PBIs have been widely constructed, including chiral (S and R isomers) guest molecules5 or host molecules6 induced assemblies of PBIs by means of hydrogen bonding,7 solvent,8 temperature9 and sonication.10 Recently, Nakashima and coworkers reported the lengths of the spacer11 tuned supramolecular chirality of PBIs. Frauenrath and coworkers reported a “twofold” odd-even effect in chirality of chiral oligopeptide-polymer-substituted perylene bisimides,12 which provided a very important design rule for supramolecular chirality.
On the other hand, carbohydrates are a natural source of chirality, possessed important effects in a wide variety of biological processes, such as cell growth regulation, differentiation, adhesion, cancer cell metastasis, cellular trafficking, inflammation by bacteria and viruses, and the immune response.13 Although, the controlled chiral self-assemblies based on carbohydrate modified perylene bisimide derivatives have been reported, they were mainly constructed by the carbohydrates modified perylene bisimide derivatives at the imide position.8a–d,14,15 However, The relationship between the supramolecular chirality and the effect of the bay-substituted groups based on perylene bisimide-carbohydrate conjugates was limited. Recently, we reported a series of perylene bisimide-carbohydrate derivatives modified in the imide and bay positions, which showed the predetermined supramolecular chirality induced by the disaccharide and the monosaccharide in the imide position of the perylene bisimides.16 Huang and coworkers reported two sugar-based perylene bisimide derivatives PTCDI-BAG and PTCDI-ClBAG with tetrachloro groups in the bay position, showed different supramolecular chirality.17 These results indicated that it is possible to adjust the supramolecular chirality by the substituted groups in the bay position of the perylene bisimide backbones.
In this paper, we reported two mannose modified perylene bisimide derivatives PBI-6Man-1 8c (Scheme 1) with triazole as the link spacer and PBI-6Man-2 16 (Scheme 1) with the amine bond as the link spacer in the bay position. It is interestingly found that opposite supramolecular chirality was achieved in aqueous solution.
The aggregation properties of PBI-6Man-2 was investigated by UV-Vis spectra. As shown in Fig. S1a,† three distinguishable absorption bands are observed between 400 and 700 nm (578 nm, 538 nm and 447 nm), and the highest intensity appears for the first band. These characteristic properties are typical for the modified perylene bisimide derivative in bay areas.18 Upon addition of water, the absorption of PBI-6Man-2 changed markedly. The intensities of the absorption bands decreased, and the absorption bands exhibited a bathochromic shift to 593 nm, 556 nm and 467 nm, respectively, and the most intensity appears at the first band (593 nm) as a result of π⋯π aggregation,18 indicating the pronounced π⋯π aggregation of PBI-6Man-2 in water. The UV-Vis spectra of PBI-6Man-1 in different volume ratios of DMSO/H2O (Fig. S1b†) showed similar changes with PBI-6Man-2. Upon addition of water, the intensities of the bands of 588 nm, 546 nm and 452 nm of PBI-6Man-1 decreased, and the absorption bands of 588 nm and 546 nm were finally bathochromic shift to 598 nm and 556 nm, respectively, but the most intensity appears at the second band (556 nm), which was different with PBI-6man-2. Compared the UV-Vis spectral data, we could conclude that PBI-6Man-1 possessed stronger π⋯π interactions than PBI-6Man-2 in water.
The distinguishable aggregation behaviours of PBI-6Man-2 in various solvents were also identified using circular dichroism (CD) spectroscopy.
As shown in Fig. 1a, no CD signal was observed for freely soluble PBI-6Man-2 in DMSO solution. However, the CD spectra changed as water was added to the solution. As 27% H2O was added to the DMSO solution, a bisignate CD signal crossing over at 511 nm was observed with two positive CD signals at ca. 387 nm and 490 nm, and with three negative CD signals at ca. 553 nm, 585 nm and 629 nm. With the further addition of H2O, the intensity of the CD signal increased. The shape of the bisignate positive/negative Cotton effect with increasing wavelength indicated that PBI-6Man-2 molecules adopt left-handed supramolecular stacking induced by the chiral D-mannose moieties.8a–d,15,17,19
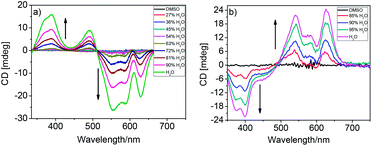 | ||
| Fig. 1 CD spectra of PBI-6Man-2 ((a), 5 × 10−5 M) and PBI-6Man-1 ((b), 6 × 10−5 M) in different volume ratios of DMSO/H2O. | ||
It is well known that the helical conformations of the π-conjugated molecules with chiral side chains afford a preferential helicity on the higher-order supramolecular assemblies.20 In the previously works of us and the other groups, different carbohydrates modified perylene bisimide derivatives had been reported, but the left-handed self-assembly induced by the D-sugar of water-soluble perylene bisimide derivative was limited.16 Considering our previous work, the six D-mannoses modified perylene bisimide derivative PBI-6Man-1 with triazole linked,8c showed right-hand chiral property in aqueous solution with two negative CD signals at ca. 376 nm and 399 nm, and with two positive CD signals at ca. 541 nm and 626 nm (Fig. 1b). We primarily assume that the dominant factor determining helicity of the left-handed conformation of the self-assembly formed by the D-mannose modified perylene bisimides PBI-6Man-2 was because of the difference of the linked spacer in the bay position between the D-mannose and the perylene backbone.
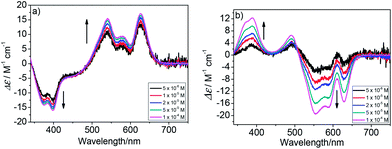
In order to investigate the differences of the π⋯π stacking interactions between compounds PBI-6Man-1 and PBI-6Man-2, concentration-dependent UV-Vis and CD spectra were performed. As shown in Fig. S2,† upon increasing the concentration, the apparent absorption coefficients (ε) of compound PBI-6man-1 showed no changes. On the other hand, the apparent absorption coefficients (ε) of compound PBI-6man-2 decreased upon increasing the concentrations. Furthermore, the concentration-dependent A0–0/A0–1 ratios of compounds PBI-6Man-1 and PBI-6Man-2 were calculated (Fig. S3†). Along with increasing of the concentrations, the A0–0/A0–1 ratios of compounds PBI-6Man-1 and PBI-6Man-2 showed no obvious changes, which were about 0.84 and 1.06, respectively. These results also indicated that PBI-6Man-1 exhibited stronger π⋯π stacking interactions than PBI-6Man-2.21 The concentration-dependent CD spectra showed similar results. Upon increasing concentrations (from 5 × 10−6 M to 1 × 10−4 M), the negative and positive Cotton effects of PBI-6Man-1 showed weak changes (Fig. 2a). But the intensities of the positive and negative Cotton effects of PBI-6Man-2 increased about four times (Fig. 2b). These results also indicated that PBI-6Man-1 showed stronger π⋯π stacking interactions than PBI-6Man-2 due to additional π⋯π stacking interactions of the triazole linker in the bay position of PBI-6Man-1.
It is well known that the π⋯π stacking interactions of perylene bisimide backbones were enthalpy driven,21 so temperature-dependent CD spectra of carbohydrates modified perylene bisimide derivatives were studied. Upon increasing temperature, significant deaggregation was observed for PBI-6Man-1 and PBI-6Man-2 (Fig. 3), however the melting temperatures showed obvious difference. Upon increasing the temperature to 80 °C, the self-assembly of PBI-6Man-1 could not completely deaggregated, the weak Cotton effects were also observed. But for compound PBI-6Man-2, the positive and negative Cotton effects disappeared at 45 °C.
 | ||
| Fig. 3 Temperature-dependent CD spectra of PBI-6man-1 (a) and PBI-6Man-2 (b) at the concentration of 5 × 10−5 M in water. | ||
Qualitative information on the relative aggregate stability was obtained by plotting the normalized difference in the CD signal at 540 nm and 550 nm (Fig. S4†). These plots clearly demonstrate that the compounds PBI-6Man-1 and PBI-6Man-2 with the melting temperatures21 of 37.7 and 28.0 °C, respectively, which showed a difference of 10 °C, indicated that the π⋯π stacking interactions of PBI-6Man-1 was stronger than PBI-6Man-2 because of additional π⋯π stacking interactions of the triazole linker in the bay position of PBI-6Man-1, similar with the results of the UV-Vis and CD spectra. These results indicated that different π⋯π stacking interactions induced difference of the chirality transfer from the chiral information of the carbohydrate to the self-assembly of the perylene bisimide backbones.11 Because of the strong π⋯π stacking interactions of PBIs for PBI-6Man-1, the intermolecular interaction between the chiral carbohydrate and the perylene backbones could not manage the arrangement of PBI units in a chiral manner with large dissymmetry, resulted PBI-6Man-1 formed right-handed supramolecular chirality. On the other the hand, the weaker π⋯π stacking interactions of PBI-6Man-2 induced chiral information smoothly transferred from the chiral carbohydrate to PBI units, resulted PBI-6Man-2 exhibited left-handed supramolecular chirality.11
Furthermore, the H-bonding interactions in the bay regions of compounds PBI-6Man-1 and PBI-6Man-2 were investigated by the FTIR spectra (Fig. 4). Compared with compound PBI-6Man-1, a strong absorption band at about 3300 cm−1 in the amide A region and a shoulder at about 1630 cm−1 in the amide I region of PBI-6Man-2 were observed, indicated H-bonding interactions between the perylene bisimide derivatives.12,22 These results indicated that the H-bonding in the bay regions played an assistant effect on the formation of the supramolecular chirality.
In summary, controllable supramolecular chirality based on the self-assembly of the perylene bisimide-carbohydrate conjugates was achieved. Under the triazole as the linker, compound PBI-6Man-1 showed right-handed chirality. On the contrary, compound PBI-6Man-2 under the amide bond as the link spacer showed left-handed chirality due to the different π⋯π stacking interactions and the additional hydrogen-bonds of the amide bond. This study provides an important design rule for controllable chiral self-assembly based on perylene bisimide-carbohydrate conjugates.
Acknowledgements
We thank NSFC (21372059), the Foundation of Hebei Education Department (YQ2013006), and the Natural Science Foundation of Hebei University for Distinguished Young Scholar (2012jq02) for financial support.Notes and references
- (a) K. R. Mackenzie, Chem. Rev., 2006, 106, 1931 CrossRef CAS PubMed; (b) K. C. Hannah and B. A. Armitage, Acc. Chem. Res., 2004, 37, 845 CrossRef CAS PubMed.
- (a) Y. Wang, J. Xu, Y. W. Wang and H. Y. Chen, Chem. Soc. Rev., 2013, 42, 2930 RSC; (b) Y. B. Lim, K. S. Moon and M. Lee, Chem. Soc. Rev., 2009, 38, 925 RSC.
- (a) H. J. Kim, T. Kim and M. Lee, Acc. Chem. Res., 2011, 44, 72 CrossRef CAS PubMed; (b) L. C. Palmer and S. I. Stupp, Acc. Chem. Res., 2008, 41, 1674 CrossRef CAS PubMed; (c) J. H. Ryu, D. J. Hong and M. Lee, Chem. Commun., 2008, 1043 RSC.
- (a) A. P. H. J. Schenning, J. V. Herrikhuyzen, P. Jonkheijm, Z. J. Chen, F. Würthner and E. W. Meijer, J. Am. Chem. Soc., 2002, 124, 10252 CrossRef CAS PubMed; (b) E. Krieg and B. Rybtchinski, Chem.–Eur. J., 2011, 17, 9016 CrossRef CAS PubMed.
- (a) C. Thalacker and F. Würthner, Adv. Funct. Mater., 2002, 12, 209 CrossRef CAS; (b) M. Kumar and S. J. George, Chem. Sci., 2014, 5, 3025 RSC.
- (a) T. Seki, A. Asano, S. Seki, Y. Kikkawa, H. Murayama, T. Karatsu, A. Kitamura and S. Yagai, Chem.–Eur. J., 2011, 17, 3598 CrossRef CAS PubMed; (b) X. Y. Lu, Z. Q. Guo, C. Y. Sun, H. Tian and W. H. Zhu, J. Phys. Chem. B, 2011, 115, 10871 CrossRef CAS PubMed; (c) P. K. Sukul, P. K. Singh, S. K. Maji and S. Malik, J. Mater. Chem. B, 2013, 1, 153 RSC.
- T. Seki, X. Lin and S. Yagai, Asian J. Org. Chem., 2013, 2, 708 CrossRef CAS PubMed.
- (a) Y. W. Huang, J. C. Hu, W. F. Kuang, Z. X. Wei and C. F. J. Faul, Chem. Commun., 2011, 47, 5554 RSC; (b) J. C. Hu, W. F. Kuang, K. Deng, W. J. Zou, Y. W. Huang, Z. X. Wei and C. F. J. Faul, Adv. Funct. Mater., 2012, 22, 4149 CrossRef CAS PubMed; (c) K. R. Wang, H. W. An, Y. Q. Wang, J. C. Zhang and X. L. Li, Org. Biomol. Chem., 2013, 11, 1007 RSC; (d) V. Stepaneko, X. Q. Li, J. Gershberg and F. Würthner, Chem.–Eur. J., 2013, 19, 4176 CrossRef PubMed.
- V. Dehm, Z. J. Chen, U. Baumeister, P. Prins, L. D. A. Siebbeles and F. Würthner, Org. Lett., 2007, 9, 1085 CrossRef CAS PubMed.
- D. M. Ke, A. L. Tang, C. L. Zhan and J. N. Yao, Chem. Commun., 2013, 49, 4914 RSC.
- (a) J. Kumar, T. Nakashima, H. Tsumatori, M. Mori, M. Naito and T. Kawai, Chem.–Eur. J., 2013, 19, 14090 CrossRef CAS PubMed; (b) J. Kumar, T. Nakashima and T. Kawai, Langmuir, 2014, 30, 6030 CrossRef CAS PubMed.
- R. Marty, R. Nigon, D. Leite and H. Frauenrath, J. Am. Chem. Soc., 2014, 136, 3919 CrossRef CAS PubMed.
- (a) D. H. Dube and C. R. Bertozzi, Nat. Rev. Drug Discovery, 2005, 4, 477 CrossRef CAS PubMed; (b) M. M. Fuster and J. D. Esko, Nat. Rev. Cancer, 2005, 5, 526 CrossRef CAS PubMed; (c) K. Ohtsubo and J. D. Marth, Cell, 2006, 126, 855 CrossRef CAS PubMed; (d) P. R. Crocker, J. C. Paulson and A. Varki, Nat. Rev. Immunol., 2007, 7, 255 CrossRef CAS PubMed; (e) F. Peri, Chem. Soc. Rev., 2013, 42, 4543 RSC.
- K. R. Wang, H. W. An, L. Wu, J. C. Zhang and X. L. Li, Chem. Commun., 2012, 48, 5644 RSC.
- Y. Huang, J. Wang and Z. Wei, Chem. Commun., 2014, 50, 8343 RSC.
- K. R. Wang, D. Han, G. J. Cao and X. L. Li, Chem.–Asian J., 2015, 10, 1204 CrossRef CAS PubMed.
- Y. Huang, J. Wang, H. Zhai, L. Zhu and Z. Wei, Soft Matter, 2014, 10, 7920 RSC.
- T. E. Kaiser, H. Wang, V. Stepanenko and F. Würthner, Angew. Chem., Int. Ed., 2007, 46, 5541 CrossRef CAS PubMed.
- (a) F. Würthner, Z. Chen, F. J. M. Hoeben, P. Osswald, C. C. You, P. Jonkheijm, J. V. Herrikhuyzen, A. P. H. J. Schenning, P. P. A. M. van der Schoot, E. W. Meijer, E. H. A. Beckers, S. C. J. Meskers and R. A. J. Janssen, J. Am. Chem. Soc., 2004, 126, 10611 CrossRef PubMed; (b) V. Dehm, Z. Chen, U. Baumeister, P. Prins, L. D. A. Siebbeles and F. Wülrthner, Org. Lett., 2007, 9, 1085 CrossRef CAS PubMed; (c) Z. Huang, E. Lee, H. J. Kim and M. Lee, Chem. Commun., 2009, 6819 RSC.
- (a) A. R. A. Palmans, J. A. J. M. Vekemans, E. E. Havinga and E. W. Meijer, Angew. Chem., Int. Ed., 1997, 36, 2648 CrossRef CAS PubMed; (b) L. Brunsveld, H. Zhang, M. Glasbeek, J. A. M. J. Vekeman and E. W. Meijer, J. Am. Chem. Soc., 2000, 122, 6175 CrossRef CAS; (c) J. van Herrikhuyzen, A. Syamakumari, A. P. H. J. Schenning and E. W. Meijer, J. Am. Chem. Soc., 2004, 126, 10021 CrossRef CAS PubMed; (d) H. Nakade, B. J. Jordan, H. Xu, G. Han, S. Srivastava, R. R. Arvizo, G. Cooke and V. M. Rotello, J. Am. Chem. Soc., 2006, 128, 14929 CrossRef PubMed; (e) G. D. Pantos, P. Pengo and J. K. M. Sanders, Angew. Chem., Int. Ed., 2007, 46, 194 CrossRef CAS PubMed.
- (a) Z. Chen, A. Lohr, C. R. Saha-Möller and F. Würthner, Chem. Soc. Rev., 2009, 38, 564 RSC; (b) Z. Chen, V. Stepanenko, V. Dehm, P. Prins, L. D. A. Siebbeles, J. Seibt, P. Marquetand, V. Engel and F. Würthner, Chem.–Eur. J., 2007, 13, 436 CrossRef CAS PubMed.
- R. Marty, H. Frauenrath and J. Helbing, J. Phys. Chem. B, 2014, 118, 11152 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional figures of UV-Vis spectra and FTIR spectra. See DOI: 10.1039/c5ra06255a |
| This journal is © The Royal Society of Chemistry 2015 |