A novel type of AIE material as a highly selective fluorescent sensor for the detection of cysteine and glutathione†
Xiaochen Tana,
Yanan Dua,
Bingchuan Yang*a and
Chen Ma*ab
aSchool of Chemistry and Chemical Engineering, Shandong University, Jinan, 250100, P. R. China
bState Key Laboratory of Bioactive Substance and Function of Natural Medicines, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100050, P. R. China. E-mail: chenma@sdu.edu.cn
First published on 17th June 2015
Abstract
A facile and efficient method was developed for the synthesis of isothiazolo[5,4-b]pyridin-3(2H)-one derivatives. This new type of AIE materials was studied through photophysical analysis, X-ray and theoretical calculations. Then, based on a ring-opening reaction, we found a useful application of this material in cysteine and glutathione detection.
Introduction
AIE (aggregation-induced emission) materials were first reported by Tang's group at 2001.1 This new kind of luminophore has plenty of advantages; unlike traditional photophysical materials, the fluorescence emission is enhanced rather than quenched after aggregation.Traditional fluorescent compounds have large π-conjugated frameworks like plates. When molecules aggregated, the fluorescence quenched. It is widely known as aggregation-caused quenching (ACQ) effect, this effect greatly limits the practical application of luminescent materials. To avoid ACQ effect, two common methods were used: adding big blocking groups or diluting the molecules in electricity conducting polymers. But the methods either increased the synthetic difficulty or reduced the efficiency. Different from the traditional fluorescent materials, AIE luminophores were proved to be the promising materials to eliminate ACQ (aggregation-caused quenching) effect. These materials have been wildly used in all kinds of areas, such as organic lighting-emitting diodes (OLEDs),2 chemosensors (CO2, HSO3− detection),3 bioprobe (DNA, RNA analysis)4 and biomedical applications.5
We have been focusing on the development of facile synthesis of AIE molecules using tandem reactions.6 Novel kind of AIE materials was synthesized and applied as cysteine and glutathione bioprobes. Cysteine and glutathione play important roles in many diseases such as liver damage, cancer and HIV infection.7 Cysteine is one of the most important amino-acids to human body, it is a precursor not only for a lot of proteins but also for glutathione.8 And glutathione levels are key index to evaluate the detoxification and redox state in organisms. High sensitive and selective fluorescent cysteine and glutathione probes are extraordinary needed in bio-research.9 As a result, several fluorescence probes were designed based on different detection mechanisms. Zhang et al.10 developed a near-infrared probe based on the conjugate addition–cyclization reaction and it can be applied in living cells. Zhou et al.11 reported a cysteine probe promoted by respond-assisted electrostatic attraction. Lee et al.12 designed an aryl-thioether substituted nitrobenzothiadiazole probe for the detection of cysteine and homocysteine.
Herein, we utilized thiosalicylic acids reacted with different amines to form new kinds of AIE materials. The reaction was processed under mild conditions, and high yields of products were obtained.
Results and discussion
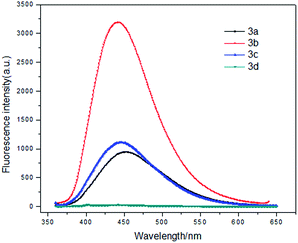
As shown in Fig. 1, four different compounds were obtained through one-pot tandem reaction. Solid-state fluorescent imagines were shown in Fig. 1.Different crystal forms were observed. All the molecules showed good optical properties except 3d which was proved to be a colorless oil. So we speculated that forming proper crystal structure was essential to lumine. Meanwhile, compound 3b showed the best fluorescence quantum yield (18%). The fluorescence quantum yields of 3a–d in THF were also detected: 3a (99%), 3b (9.0%), 3c (2.8%) and 3d (99%) (Fig. 2).
X-ray crystallographic analysis was shown in Fig. 3. It configured the structure of 3a. Meanwhile, HOMOs/LUMOs of 3a–d were also calculated by Gaussian 03 program using the B3LYP method with the 6-31G basis set (Scheme 1).13 Clear intramolecular charge transfer (ICT) effects were observed in 3b and 3d. As a result, 3b gave better luminescence than the other molecules. Compound 3d was oil under room temperature, which made 3d lose AIE characteristic (Fig. 4).
 | ||
| Scheme 1 Synthesis and absolute quantum yields determined by a calibrated integrating sphere system of fused isothiazolidin-3-ones 3. | ||
 | ||
| Fig. 4 HOMO/LUMO imagines of 3a–d calculated by Gaussian 03 program using the B3LYP method with the 6-31G basis set.13 | ||
Compound 3b showed the best optical characteristics. The DLS (Dynamic Light Scattering) radiuses of 3a–c were also measured (Fig. S3 in the ESI†). The results confirmed that compound 3a–c were aggregated into sub-micron particles in THF/water solutions.
What is more, the AIE properties of 3a and 3b were determined in THF/water (from 100/0 to 1/99, v/v) shown in Fig. 5 and 6. An interesting optical phenomenon was found. The compounds showed ACQ effect at first, then AIE effect was observed after adding large amount of water. In pure THF, molecule 3b gave luminescence at 400 nm. Then the fluorescence quenched after water fraction raised from 0% to 60%. When water fraction increased to 90%, the molecules began to aggregate into sub-micron particles. After that, a new fluorescence emission was observed at 450 nm. The emission was both bathochromic-shifted and enhanced after aggregation.
 | ||
| Fig. 5 Fluorescence emission spectra of 3a (excitation at 330 nm) in THF/water mixtures with different water fractions (fw). | ||
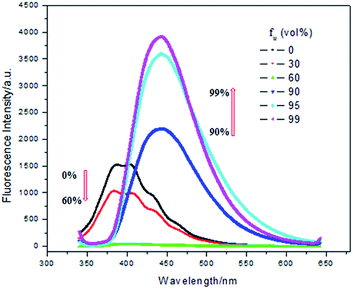 | ||
| Fig. 6 Fluorescence emission spectra of 3b (excitation at 330 nm) in THF/water mixtures with different water fractions (fw). | ||
After all the photophysical measurements were taken, we developed compound 3b into the cysteine and glutathione bioprobes based on a ring-opening reaction. The detection system was a THF/water solution buffered by 20 mM HEPES at pH 7.2. The probes were existed as sub-micron particles in the system. They were highly fluorescent at first. After the addition of cysteine, the fluorescence intensity decreased dramatically. It was a highly selective analysis system.
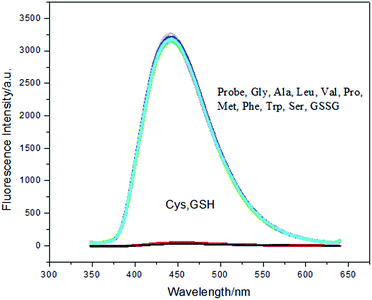
The selectivity of probe 3b for Cys (cysteine) and GSH (glutathione) was compared to the other amino acids, such as Gly (glycine), Ala (alanine), Leu (leucine), Val (valine), Pro (proline), Met (methionine), Phe (phenylalanine), Trp (tryptophan), Ser (serine), GSSG (oxidized glutathione) shown in Fig. 7. Only cysteine and GSH (glutathione) could make the probe 3b quench. The analytical system was proved to be highly selective.
Fluorescence titration of cysteine detection was taken in THF/water (1/99, v/v) buffered by 20 mM HEPES. The probe solution (3b, 1 × 10−5 M) was prepared according to the methods described in the ESI.† Then the titration was proceeded by adding cysteine solution (1 × 10−3 M, 10 μL each time) into the probe solution (1 × 10−5 M, 1 mL). Upon the addition of cysteine, the emission peak gradually decreased.
The fluorescence emission curve was shown in Fig. 8 at pH 7.20. And titration linear for Cys at the concentration between 0 μM and 7 μM was shown in Fig. 9 (R = 0.99987, n = 7). The linear regression equation was calculated to be 1 − I/I0 = −0.000129916 + 0.12986X. According to IUPAC, the detection limit was calculated as 0.5 μM and a relative standard deviation of 0.9% was measured for 5.0 μM Cys. And the fluorescence titration of GSH (glutathione) was also proceeded under the same condition. The result was shown in Fig. 10.
 | ||
| Fig. 8 Fluorescence emission (excitation at 330 nm, slit: 5 nm/10 nm) of 3b (1 × 10−5 M) was titrated with Cys (0–0.9 equiv.) in THF/water solution (1/99, v/v) buffered by 20 mM HEPES at pH 7.2. | ||
 | ||
| Fig. 10 Fluorescence emission (excitation at 330 nm, slit: 5 nm/10 nm) of 3b (1 × 10−5 M) was titrated with GSH (0–0.9 equiv.) in THF/water solution (1/99, v/v) buffered by 20 mM HEPES at pH 7.2. | ||
Conclusions
A series of AIE compounds were synthesized through one-pot tandem reaction. Different optical properties were observed when R group changed. Only the materials which have solid-state showed good AIE characteristics. An interseting bathochromic-shifted and enhanced luminescence were also detected. At last, we developed a new kind of cysteine and glutathione probes based on a ring-opening reaction. The detection system was proved to be highly selective.Experimental section
All reagents and solvents were pure analytical-grade materials purchased from commercial sources and were used without further purification, if not stated. All reactions were monitored by thin-layer chromatography (TLC). 1H NMR spectra were recorded on a Bruker Avance 400 or 300 spectrometer at 400 or 300 MHz, using CDCl3 as solvent and tetramethylsilane (TMS) as internal standard. 13C NMR spectra were run in the same instrument at 100 or 75 MHz. HRMS spectra were determined on a Q-TOF6510 spectrograph (Agilent).To a solution of 2-mercaptonicotinic acid (0.31 g, 2 mmol) in CH2Cl2 (20 mL) thionyl chloride (0.3 mL) was added, the solution was refluxed for 2.5 h (oil bath). The mixture was evaporated in vacuo, and the solvent and thionyl chloride were removed, yellow solid was obtained. The yellow solid was dissolved by CH2Cl2 (20 mL), then cyclohexylamine (0.20 g, 2 mmol) dissolved in 10 mL CH2Cl2 was added dropwise to the ice bath solution. The mixture was stirred overnight, then H2O (100 mL) was added and the mixture was filtered and extracted with CH2Cl2 (3 × 50 mL). The combined organic layers were washed by chilled water (3 × 100 mL), dried by Na2SO4, filtered, and evaporated in vacuo. The crude product was purified by flash chromatography on silica gel (hexane/EtOAc = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Compound 3a was obtained as white plate crystals (0.11 g, 47%).
1). Compound 3a was obtained as white plate crystals (0.11 g, 47%).
2-Cyclohexylisothiazolo[5,4-b]pyridin-3(2H)-one (3a)
White solid (47%). 1H NMR (300 MHz, CDCl3) δ 8.73 (dd, 1H, J = 4.5, 1.5 Hz), 8.29–8.26 (m, 1H), 7.37–7.32 (m, 1H), 4.69–4.59 (m, 1H), 2.07 (d, 2H, J = 11.4 Hz), 1.90 (d, 2H, J = 13.2 Hz), 1.74 (d, 1H, J = 13.2 Hz), 1.65–1.40 (m, 4H), 1.30–1.16 (m, 1H). 13C NMR (75 MHz, CDCl3) δ 163.1, 162.4, 153.2, 134.7, 120.6, 120.3, 53.2, 32.9, 25.6, 25.2. HRMS calcd for C12H14N2OS, 235.0900; found, 235.1052.2-(3,4,5-Trimethoxyphenyl)isothiazolo[5,4-b]pyridin-3(2H)-one (3b)
Yellow solid (43%). 1H NMR (300 MHz, CDCl3) δ 8.82 (dd, 1H, J = 4.8, 1.5 Hz), 8.34 (dd, 1H, J = 7.8, 1.5 Hz), 7.42 (dd, 1H, J = 8.0, 1.5 Hz), 6.91 (s, 1H), 3.90 (s, 6H), 3.88 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 162.6, 161.9, 154.1, 153.6, 137.6, 135.2, 132.0, 121.1, 119.7, 103.1, 61.0, 56.3. HRMS calcd for C15H14N2O4S, 319.0708; found, 319.0751.Methyl 2-(3-oxoisothiazolo[5,4-b]pyridin-2(3H)-yl)benzoate (3c)
White solid (45%). 1H NMR (300 MHz, DMSO-d6) δ 8.94 (dd, 1H, J = 7.6, 1.6 Hz), 8.38 (dd, 1H, J = 8.0, 1.6 Hz), 7.98 (dd, 1H, J = 8.0, 1.6 Hz), 7.83–7.78 (m, 1H), 7.67–7.59 (m, 3H), 3.64 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 165.0, 162.7, 161.9, 154.6, 135.3, 134.5, 133.6, 131.0, 129.7, 129.4, 129.2, 121.7, 117.9, 52.3. HRMS calcd for C14H10N2O3S, 287.0446; found, 287.0551.2-(Naphthalen-2-yl)isothiazolo[5,4-b]pyridin-3(2H)-one (3d)
Colorless oil (38%). 1H NMR (300 MHz, DMSO-d6) δ 8.98 (dd, 1H, J = 4.6, 1.6 Hz), 8.45 (dd, 1H, J = 7.8, 1.5 Hz), 8.16–8.10 (m, 2H), 7.78 (d, 1H, J = 6.6 Hz), 7.70–7.56 (m, 5H), 3.31 (s, 1H). 13C NMR (100 MHz, CDCl3) δ 163.4, 162.7, 155.1, 135.9, 134.4, 132.4, 130.6, 129.0, 128.5, 128.0, 127.4, 126.2, 123.1, 122.3, 118.6. HRMS calcd for C16H10N2OS, 279.0547; found, 279.0579.Acknowledgements
We are grateful to the National Science Foundation of China (Grant nos 21172131, 21273131) and State Key Laboratory of Bioactive Substance and Function of Natural Medicines, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College (no GTZK201405) for financial support of this research.References
- (a) J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu and B. Z. Tang, Chem. Commun., 2001, 1740–1741 RSC; (b) B. Z. Tang, X. Zhan, G. Yu, P. P. S. Lee, Y. Liu and D. Zhu, J. Mater. Chem., 2001, 11, 2974–2978 RSC.
- (a) Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Commun., 2009, 4332–4353 RSC; (b) J. Chen, C. C. W. Law, J. W. Y. Lam, Y. Dong, S. M. F. Lo, I. D. Williams, D. Zhu and B. Z. Tang, Chem. Mater., 2003, 15, 1535 CrossRef CAS; (c) G. Yu and B. Z. Tang, et al., J. Am. Chem. Soc., 2005, 127, 6335 CrossRef CAS PubMed; (d) Z. J. Zhao, J. L. Geng, Z. F. Chang, S. M. Chen, C. M. Deng, T. Jiang, W. Qin, J. W. Y. Lam, H. S. Kwok, H. Y. Qiu, B. Liu and B. Z. Tang, J. Mater. Chem., 2012, 22, 11018 RSC; (e) J. Huang, X. Yang, J. Wang, C. Zhong, L. Wang, J. Qin and Z. Li, J. Mater. Chem., 2012, 22, 2478 RSC; (f) Z. J. Zhao, S. M. Chen, J. W. Y. Lam, Z. M. Wang, P. Lu, F. Mahtab, H. H. Y. Sung, I. D. Williams, Y. G. Ma, H. S. Kwok and B. Z. Tang, J. Mater. Chem., 2011, 21, 7210 RSC; (g) J. Huang, N. Sun, J. Yang, R. Tang, Q. Li, D. Ma, J. Qin and Z. Li, J. Mater. Chem., 2012, 22, 12001 RSC.
- (a) Y. Liu, Y. Tang, N. N. Barashkov, I. S. Irgibaeva, J. W. Y. Lam, R. Hu, D. Birimzhanova, Y. Yu and B. Z. Tang, J. Am. Chem. Soc., 2010, 132, 13951–13953 CrossRef CAS PubMed; (b) C. Y.-S. Chung and V. W.-W. Yam, J. Am. Chem. Soc., 2011, 133, 18775 CrossRef CAS PubMed; (c) Y. Liu, Y. H. Tang, N. N. Barashkov, I. S. Irgibaeva, J. W. Y. Lam, R. R. Hu, D. Birimzhanova, Y. Yu and B. Z. Tang, J. Am. Chem. Soc., 2010, 132, 13951 CrossRef CAS PubMed; (d) C. Li, T. Wu, C. Hong, G. Zhang and S. Liu, Angew. Chem., Int. Ed., 2012, 51, 455 CrossRef CAS PubMed; (e) M. Wang, G. Zhang, D. Zhang, D. Zhu and B. Z. Tang, J. Mater. Chem., 2010, 20, 1858 RSC; (f) J. Wu, W. Liu, J. Ge, H. Zhang and P. Wang, Chem. Soc. Rev., 2011, 40, 3483 RSC.
- (a) Y. Dong, J. W. Y. Lam, A. Qin, Z. Li, J. Liu, J. Liu, J. Sun, Y. Dong and B. Z. Tang, Chem. Phys. Lett., 2007, 446, 124–127 CrossRef CAS PubMed; (b) C. W. T. Leung, Y. Hong, S. Chen, E. Zhao, J. W. Y. Lam and B. Z. Tang, J. Am. Chem. Soc., 2012, 135, 62 CrossRef PubMed; (c) H. Shi, J. Liu, J. Geng, B. Z. Tang and B. Liu, J. Am. Chem. Soc., 2012, 134, 9569 CrossRef CAS PubMed; (d) H. Lu, F. Su, Q. Mei, Y. Tian, W. Tian, R. H. Johnson and D. R. Meldrum, J. Mater. Chem., 2012, 22, 9890 RSC; (e) Y. Yu, C. Feng, Y. Hong, J. Liu, S. Chen, K. M. Ng, K. Q. Luo and B. Z. Tang, Adv. Mater., 2011, 23, 3298 CrossRef CAS PubMed.
- (a) X. Zhang, X. Zhang, L. Tao, Z. Chi, J. Xu and Y. Wei, J. Mater. Chem. B, 2014, 2(28), 4398–4414 RSC; (b) X. Li, X. Zhang, Z. Chi, X. Chao, X. Zhou, Y. Zhang and J. Xu, Anal. Methods, 2012, 4(10), 3338–3343 RSC; (c) C. Li, T. Wu, C. Hong, G. Zhang and S. Liu, Angew. Chem., 2012, 124(2), 470–474 CrossRef PubMed.
- (a) A. P. Huang, Y. M. Chen, Y. G. Zhou, W. Guo, X. D. Wu and M. Chen, Org. Lett., 2013, 15, 5480–5483 CrossRef CAS PubMed; (b) Y. M. Zhao, Y. M. Wu, J. Jia, D. J. Zhang and C. Ma, J. Org. Chem., 2012, 77, 8501–8506 CrossRef CAS PubMed; (c) B. C. Yang, X. Y. Niu, Z. X. Huang, C. H. Zhao, Y. Liu and C. Ma, Tetrahedron, 2013, 69, 8250–8254 CrossRef CAS PubMed; (d) B. C. Yang, X. C. Tan, R. Y. Guo, S. W. Chen, Z. Y. Zhang, X. L. Chu, C. X. Xie, D. J. Zhang and C. Ma, J. Org. Chem., 2014, 79, 8040–8048 CrossRef CAS PubMed.
- (a) D. M. Townsend, K. D. Tew and H. Tapiero, Biomed. Pharmacother., 2003, 57, 145 CrossRef CAS; (b) J. M. Estrela, A. Ortega and E. Obrador, Crit. Rev. Clin. Lab. Sci., 2006, 43, 143 CrossRef CAS PubMed; (c) B. Helbling, J. Von Overbeck and B. H. Lauterburg, Eur. J. Clin. Invest., 1996, 26, 38 CrossRef CAS.
- W. Wang, O. Rusin, X. Xu, K. K. Kim, J. O. Escobedo, S. O. Fakayode, K. A. Fletcher, M. Lowry, C. M. Schowalter, C. M. Lawrence, F. R. Fronczek, I. M. Warner and R. M. Strongin, J. Am. Chem. Soc., 2005, 127(45), 15949–15958 CrossRef CAS PubMed.
- X. Chen, Y. Zhou, X. Peng and J. Yoon, Chem. Soc. Rev., 2010, 39(6), 2120–2135 RSC.
- J. J. Zhang, J. X. Wang, J. T. Liu, L. L. Ning, X. Y. Zhu, B. F. Yu, X. Y. Liu, X. J. Yao and H. X. Zhang, Anal. Chem., 2015, 87, 4856–4863 CrossRef CAS PubMed.
- X. Zhou, X. J. Jin, G. Y. Sun, D. H. Li and X. Wu, Chem. Commun., 2012, 48, 8793–8795 RSC.
- D. Lee, G. Kim, J. Yin and J. Yoon, Chem. Commun., 2015, 51, 6518–6520 RSC.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery Jr T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyata, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. AlLaham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez and J. A. Pople, Gaussian 03, Revision B.01, Gaussian, Pittsburgh, PA, 2003 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1051210. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra06244f |
| This journal is © The Royal Society of Chemistry 2015 |